(People’s Daily Health Client Zhou Xuejin) According to incomplete statistics from the People’s Daily Health Client, 6 innovative medical devices have been approved for the market since July, covering intestinal polyps diagnosis and treatment, implantable left ventricular assist system, etc. field. Where are these 6 innovative medical devices “new”? What new options will it bring to the treatment of related diseases?
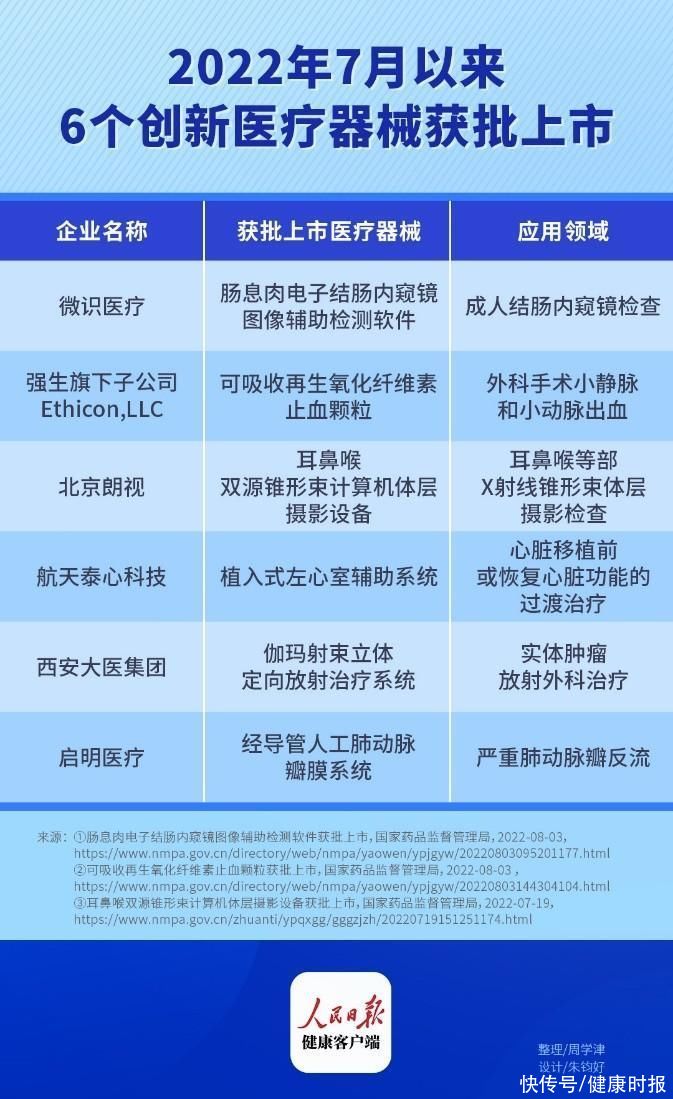
Weishi Medical: The first medical device software in China that uses deep learning technology to assist polyp detection
The “Intestinal Polyp Electronic Colonoscopy Image-Assisted Detection Software” produced by Chengdu Weishi Medical Equipment Co., Ltd. can be used in conjunction with electronic colonoscopy to help doctors find Identifying the location of suspected polyps is conducive to earlier detection of precancerous lesions of rectal cancer, thereby reducing the incidence and mortality of rectal cancer.
According to the State Drug Administration, this is the first medical device software in China that uses deep learning technology to assist in the detection of polyps in endoscopic images. It adopts small sample deep learning technology and local labeling technology. For the selection and development of the algorithm model framework, the overall performance of the algorithm is not completely dependent on the increase of training data, and can achieve high performance, strong generalization and robustness under smaller samples.
Ethicon, LLC, a subsidiary of Johnson & Johnson: the first granular regenerated oxidized cellulose hemostatic product
The product produced by Ethicon, LLC, a subsidiary of Johnson & Johnson Absorbable regenerated oxidized cellulose hemostatic particles”, suitable for surgery or endoscopic surgery (except ophthalmology, neurosurgery, urology), when ligation or other traditional control methods are not applicable or ineffective, as an auxiliary control capillary, Bleeding from venules and arterioles.
It is reported that this product is the first granular regenerated oxidized cellulose hemostatic product. Effective and uniform spray, easy to use in surgical or endoscopic procedures.
Beijing Longview: the world’s first oral CBCT product with dual sources and dual detectors
“Ear, Nose and Throat” produced by Beijing Longview Instrument Co., Ltd. Dual-source cone-beam computed tomography equipment”, which can be used for X-ray cone-beam tomography of the ear, nose, throat airway, oral and maxillofacial region. This is the world’s first dual-source, dual-detector oral CBCT product. It uses dual-source imaging technology, uses a small focus, high-power X-ray tube head and high-resolution detectors to improve the spatial resolution. Based on imaging needs, we can provide X-ray cone beam tomography for ENT diseases.
Aerospace Taixin Technology: The first implantable magnetic fluid suspension left ventricular assist system in China using magnetic suspension technology
Aerospace Taixin Technology Co., Ltd. The “implantable left ventricular assist system” produced by the company is used in conjunction with specific artificial blood vessels to provide mechanical support for the blood circulation of patients with advanced refractory left heart failure, and is used for transitional treatment before heart transplantation or restoring cardiac function. It is intended for use by medical institutions with heart transplant conditions and comprehensive post-operative care capabilities. Medical staff, out-of-hospital nurses and patients must pass corresponding training. Contraindicated in patients with intolerance to anticoagulant therapy.
This product adopts the main core technologies such as pump-machine integrated design, magnetic-fluid suspension, internal flow channel optimization, and double-redundancy of the drive, all of which have independent intellectual property rights. The key technical indicators of the implantable magnetic fluid suspension left ventricular assist system have reached the same international level.
Xi’an Great Medical Group: The first image-guided gamma ray stereotactic radiotherapy equipment in China
The “Gamma” produced by Xi’an Great Medical Group Co., Ltd. “Ma Beam Stereotactic Radiation Therapy System” for image-guided head, radiosurgery, and body stereotaxic radiation therapy for solid tumors and lesions. It integrates KV-level CBCT image guidance technology and real-time orthogonal imaging image guidance technology, and has independent intellectual property rights. It is the first image-guided gamma ray stereotactic radiotherapy equipment in China.
Qiming Medical: A New Option for the Treatment of Severe Pulmonary Valve Regurgitation
The innovative product “Transcatheter Prosthetic Pulmonary Valve” produced by Hangzhou Qiming Medical Equipment Co., Ltd. system”, which consists of the pulmonary valve and the delivery system. The pulmonary valve consists of a self-expanding nickel-titanium alloy stent, leaflets and skirts made of porcine pericardium, and sutures. The design of the pulmonary valve can make the anchoring of the valve stent more stable, suitable for patients with a wider range of pulmonary valve annulus of different anatomical shapes, while making the stent delivery safer and blood flow smoother. The design of the delivery system allows the valve to be compressed uniformly, improving loading efficiency and reducing the risk of radial collapse of the valve upon release.
This product is suitable for postoperative congenital heart disease patients with severe pulmonary regurgitation (≥3+) with native right ventricular outflow tract (age ≥12 years old, weight ≥30kg), and must meet certain requirements. Conditions of clinical use (see product scope for details). It is understood that the launch of the product will bring new treatment options to patients.