This article is reproduced from [China News];
China News, August 10th. Recently, the Ministry of Industry and Information Technology, the National Health Commission and other four departments jointly issued a notice to deploy and strengthen the concentration of drugs in shortage and national organizations. Monitoring of production and reserve of selected drugs in procurement. what does that mean? Will there be shortages of medicines in the future?

Photo by Zhang Tianfu, a reporter from Xinshe in the data picture
What is a shortage of medicines?
The “Notice on Printing and Distributing the National Drug Shortage List” issued by 12 departments including the National Health and Health Commission, the National Development and Reform Commission, and the Ministry of Industry and Information Technology in 2020 shows that there are 6 varieties of the national drug shortage list, focusing on In response to the shortage of production and supply side, to ensure the supply of medicines.
In addition, the National Key Monitoring List of Clinically Necessary and Shortaged Drugs has a total of 57 varieties, focusing on drugs that are clinically necessary, irreplaceable or irreplaceable, and have the risk of supply shortage. , early warning, and timely measures to prevent shortages.
It is worth noting that dynamic adjustments have been implemented in both the catalogue of drug shortage monitoring varieties and the catalogue of monitored enterprises.
The four-department notice also pointed out that in terms of monitoring varieties, the National Health and Health Commission announced the National Shortage Drug List, the National Clinically Necessary Drugs in Shortage Key Monitoring List, and the National Medical Insurance Bureau announced the national organization. Selected drugs for centralized procurement of drugs. Dynamic adjustment of the monitoring variety catalog implementation .
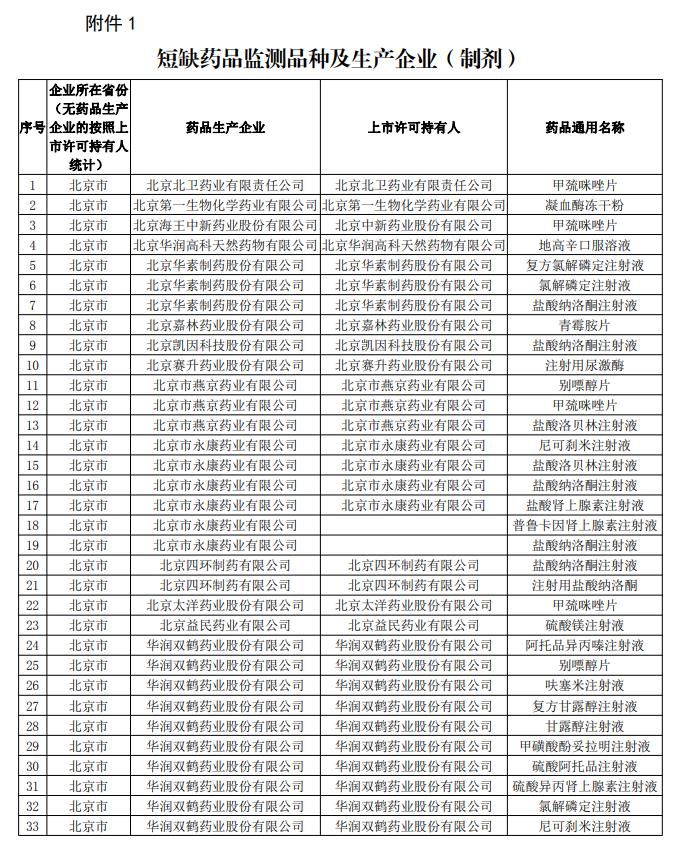
A screenshot of the section “Monitoring Varieties of Shortaged Drugs and Manufacturers (Preparations)” in the notice.
What have you done over the years to ensure that there is no shortage of medicines in short supply?
In fact, in order to ensure the supply and production of drugs in short supply, many departments have introduced relevant measures in recent years.
In 2021, the General Office of the State Council will issue the “14th Five-Year Plan for National Medical Insurance”. Among them, it is proposed to improve the monitoring, early warning and graded response system for drugs in shortage, strengthen the enforcement of illegal acts such as monopoly of raw materials, and further ensure the supply and price of drugs in shortage.
The planning is clear, and the emergency reserve, inventory and production capacity reporting system for the winning production enterprises will be gradually established to ensure the centralized procurement of drug supply. Support the chain, professional, and digital development of pharmacies, and give better play to the unique advantages of pharmacies and the role of pharmacists. Relying on the national unified medical security information platform, it supports the circulation of electronic prescriptions.
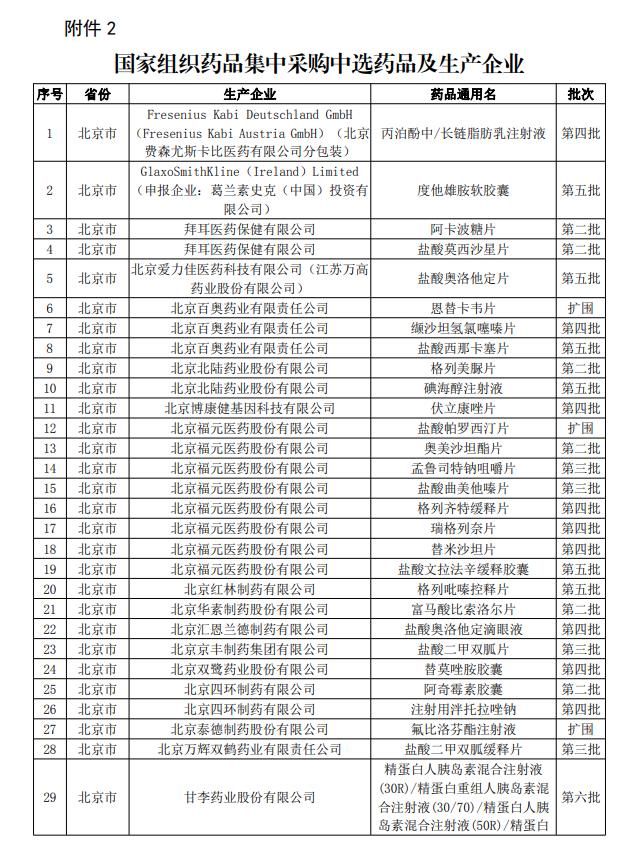
A screenshot of “Selected Drugs and Manufacturers in Centralized Drug Procurement organized by the State”.
In November 2021, the State Food and Drug Administration developed and constructed the information collection module for the production supply and discontinuation report of shortage drugs in the drug information collection platform.
In January of this year, the “14th Five-Year Plan for the Development of Pharmaceutical Industry” jointly issued by the Ministry of Industry and Information Technology, the National Development and Reform Commission and other nine departments proposed to enhance the supply guarantee capability of drugs that are prone to shortage. Focusing on essential medicines, children’s medicines, emergency medicines, etc. Dynamically adjust the national list of drugs in short supply and the key monitoring list of clinically necessary drugs that are prone to shortage, strengthen the production and supply chain monitoring and early warning of drugs prone to shortage, and establish a supply and demand docking platform for drugs prone to shortage . Support the development of a drug supply guarantee consortium, expand the coverage of small-variety drugs (drugs in short supply) centralized production bases, deepen supply chain collaboration, and promote the integrated development of key varieties of APIs and preparations.

Data map photo by Guo Jia
These situations may be interviewed and rectified in the future
In addition to the above measures, how to monitor the shortage of medicines in a timely manner has recently been answered.
On August 9, the website of the Ministry of Industry and Information Technology published the notice of the four departments on strengthening the monitoring of the production and reserve of drugs in short supply and the centralized procurement of drugs organized by the state.
Among them, it is required to give full play to the role of the provincial-level consultation and linkage mechanism, further strengthen information communication and sharing, strengthen monitoring and early warning, improve the management measures for grading and responding to shortage of drugs, guide and supervise enterprises to fulfill their information reporting obligations, and continuously improve drugs. Production and supply security capabilities to better meet the health needs of the people.
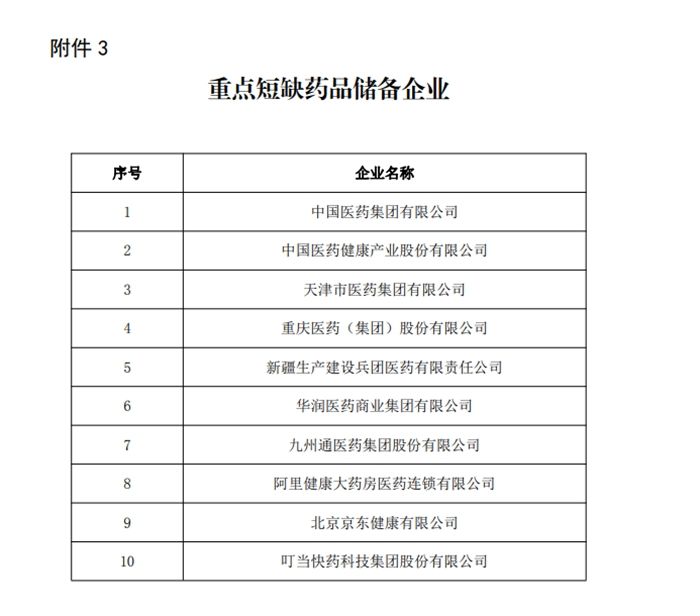
The key shortage drug reserve enterprises announced by the notice
The notice is clear, before the 10th of each month, there will be shortages Drug production enterprises and selected drug manufacturers in centralized procurement shall, through the “Monitoring and Early Warning Platform for Drug Shortage Production and Supply”, fill in the “Monitoring Report on Production and Supply of Drugs in Shortage of Pharmaceutical Industry Enterprises” and “Monitoring Report on Production and Supply of National Centralized Procurement Drugs”; key shortage drug reserves Through the “National Pharmaceutical Reserve Management Information System”, the enterprise fills in the “Statistical Form for the Quantity of Inventory of Drugs in Shortage”.
The notice requires that the production enterprises of drugs in shortage and selected drugs in centralized procurement are the first responsible person for the reporting of production and reserve monitoring information. It is necessary to establish and improve the monitoring information reporting work system; Fill in the production and reserve data in a timely manner, without delay or refusal to report; cooperate with relevant departments in the investigation of drug shortage incidents, and provide the information required for the investigation; set up production and supply inventory safety warning lines and prepare response plans.
The notice also calls for strengthening supervision and management. The local industry and information technology department, together with the relevant departments, is responsible for the production and reserve monitoring of the drugs in shortage and the selected drugs in the centralized procurement in the region, organizes enterprises to submit relevant data, audits the integrity and accuracy of the data, and strengthens the supervision and inspection of drug quality. Supervise and urge production enterprises to implement their responsibilities for sufficient supply and discontinuation reporting in accordance with the purchase agreement. For enterprises that fail to comply with the requirements, they will be interviewed and required to rectify within a time limit as the case may be. (Zhongxin Finance)