On July 25, the State Food and Drug Administration, in accordance with the relevant provisions of the “Drug Administration Law” and in accordance with the special drug approval procedures Emergency review and approval, and conditionally approved the registration application of Henan True Biotechnology Co., Ltd. for the addition of azvudine tablets for the treatment of new coronavirus pneumonia.
This product is an oral small molecule novel coronavirus pneumonia treatment drug independently developed by my country. On July 20, 2021, the State Food and Drug Administration has conditionally approved this product in combination with other reverse transcriptase inhibitors for the treatment of adult HIV-1 infected patients with high viral load. This is a conditional approval of an additional indication for the treatment of adult patients with common novel coronavirus pneumonia (COVID-19). Patients should strictly follow the instructions for medication under the guidance of a physician.
The State Food and Drug Administration requires the marketing authorization holder to continue to carry out relevant research work, complete the conditional requirements within a time limit, and submit the follow-up research results in a timely manner.
[Previously reported]
my country’s first anti-coronavirus oral drug arrives
Azvudine Tablets in the treatment of new coronary pneumonia indication registration phase III clinical trial
Main efficacy The indicators meet expectations
Top News·Henan Daily reporter Ke Yang Li Xiaomin
Approved to carry out phase III clinical trials for more than two years Azvudine is expected to become my country’s first oral anti-new crown drug with completely independent intellectual property rights!
On July 15, Henan Real Biotechnology Co., Ltd. (hereinafter referred to as “Real Bio”) announced that it has recently formally submitted azvudine treatment to the State Drug Administration. Marketing applications for novel coronavirus indications.
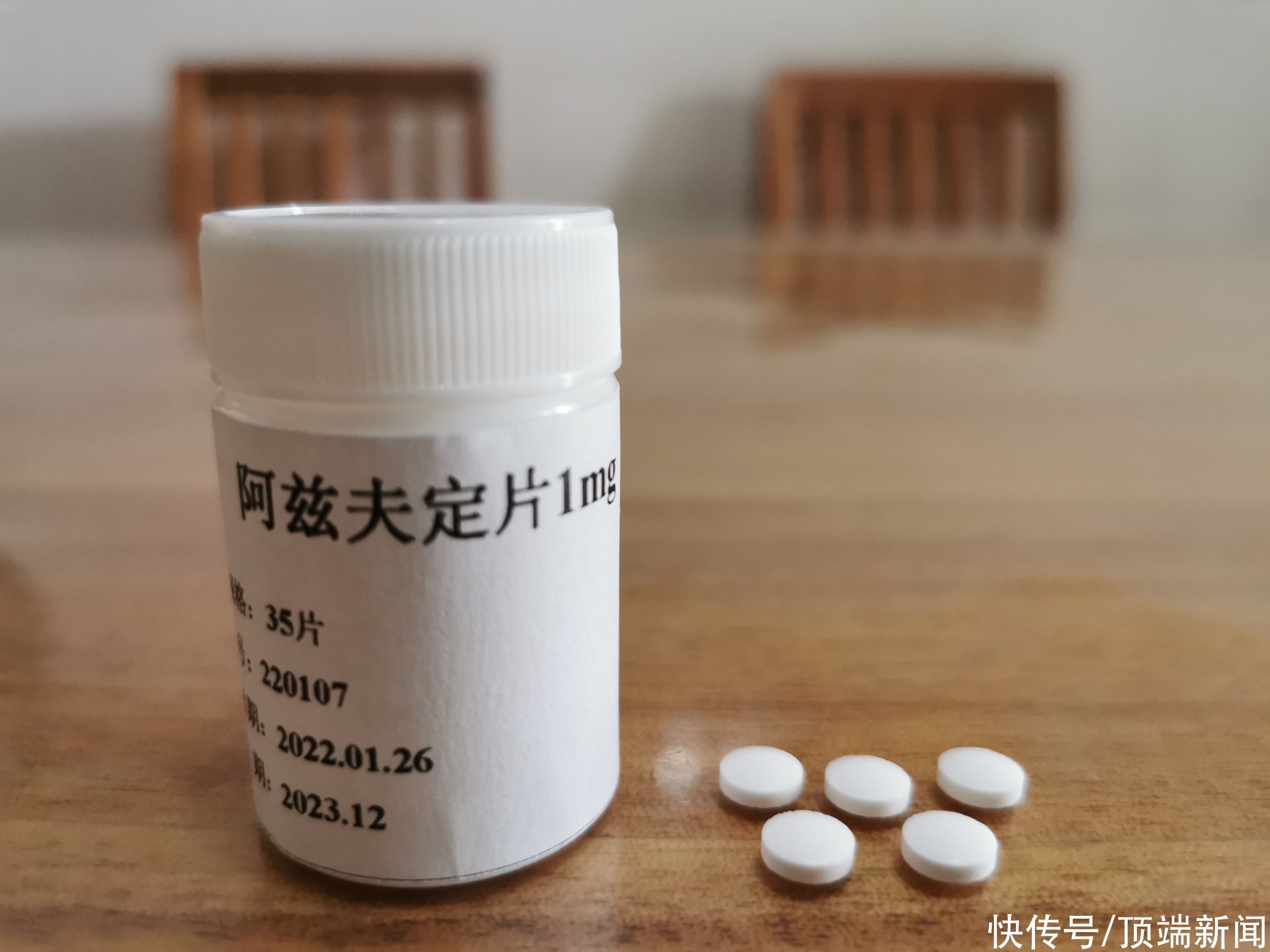
Since April 2020, Azvudine has been approved for development at home and abroad Phase III clinical trials. The clinical trial results showed:
(1) Significantly improve clinical symptoms: Azvudine Tablets can significantly shorten the symptom improvement time in patients with moderate novel coronavirus infection pneumonia, improve clinical symptoms The proportion of patients whose symptoms improved and achieved clinically superior results. The proportion of subjects whose clinical symptoms improved on the 7th day after the first administration was 40.43% in the Azvudine group and 10.87% in the placebo group (P value < 0.001), and the median time to clinical improvement of subjects in the Azvudine group There was a very significant statistical difference with the placebo group (P value < 0.001). (PPS set)
(2) Inhibition of the new coronavirus: Azvudine has the activity of inhibiting the new coronavirus, and the virus clearance time is about 5 days.
(3) Safety: Azvudine tablets were generally well tolerated, and there was no significant difference in the incidence of adverse events between the Azvudine group and the placebo group. No increased subject risk.
It is understood that Azvudine is the world’s first innovative dual-target anti-AIDS drug, and it has applied for and authorized patents in China, the United States and other countries. As an antiviral small molecule oral drug, Azvudine has a broad-spectrum inhibitory effect on RNA virus replication, and the new coronavirus is a virus with RNA as the genetic material, so the drug has an inhibitory effect on the new coronavirus.
Dr. Du Jinfa, Chief Scientist of Real Biology, said: “We are very happy to usher in this important moment and look forward to the early approval of Azivudin, which will help prevent and control the epidemic in China and around the world. Provide strength.”

Azvudine drug inventor, former Henan Normal University Professor Chang Junbiao, President of the University and now Deputy Secretary and Vice President of Zhengzhou University, said: “As a nucleoside analog that inhibits viral RNA-dependent RNA polymerase (RdRp), Azvudine can specifically act on the new coronavirus RdRp. Thereby inhibiting virus replication, its drug targeting is strong.”
Jiang Jiandong, academician of the Chinese Academy of Engineering and dean of the Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, said: “Azef It must have obvious anti-COVID-19 effect and is effective for clinically mild and severe patients. Azvudine treats COVID-19 through a molecular mechanism that treats both the symptoms and the symptoms, which is its unique biological feature.”