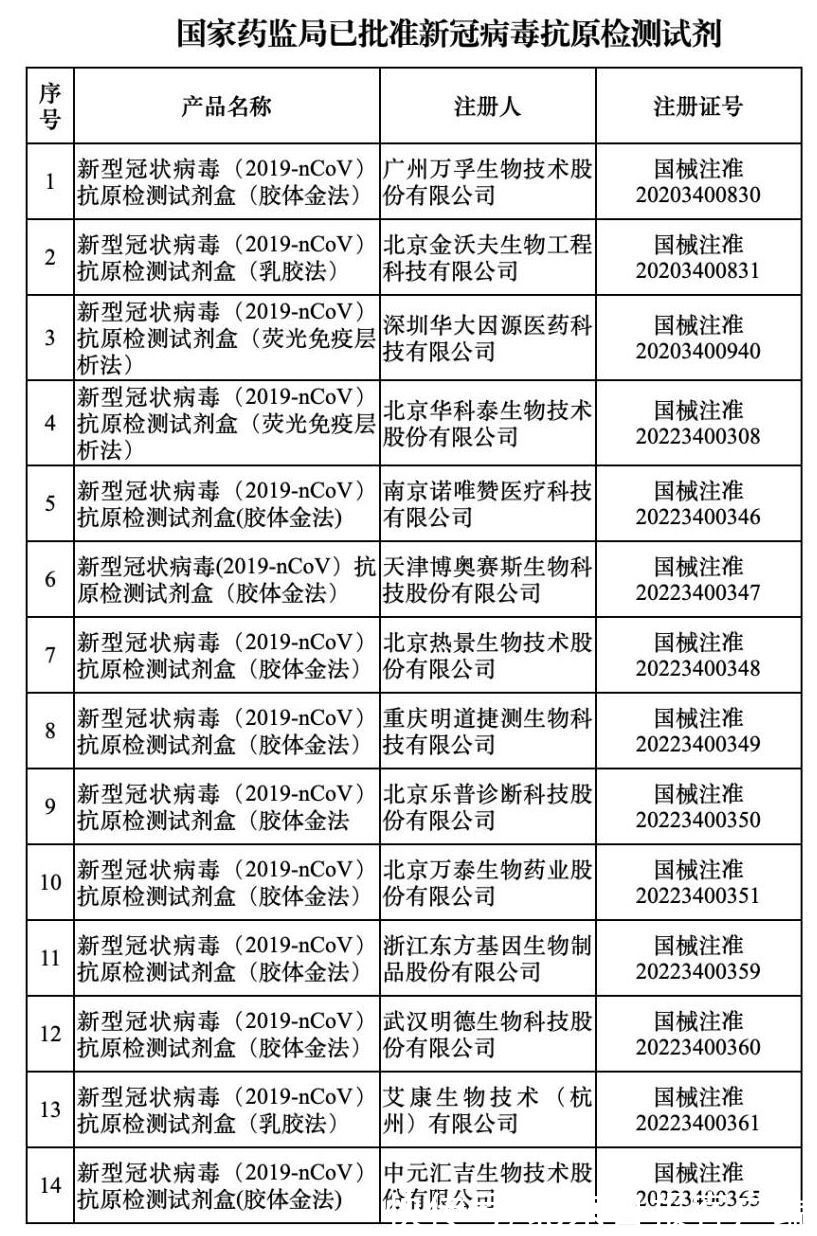
The reporter learned from the State Food and Drug Administration today: On March 17, after review by the State Food and Drug Administration, a new coronavirus antigen detection reagent product was approved. As of March 17, the State Food and Drug Administration has approved 14 novel coronavirus antigen detection reagent products.
The Novel Coronavirus Antigen Detection Reagent is suitable for the “New Coronavirus Antigen Detection Application Scheme (Trial)” (Comprehensive Joint Prevention and Control Mechanism) [2022] No. 21) the specified crowd.
Drug supervision and administration departments will strengthen post-market supervision of related products to protect the safety of patients using devices.
SourcePeople’s Daily Client< /span> | Author Shen Shaotie
Editor Gao Shanshan
Process editor Weili Liu