On April 6, Kintor-B (09939.HK) announced that its new crown treatment drug Prokluamide had completed the Phase III clinical trial. Under relevant conditions, the test patients achieved a 100% protection rate. After soaring 229% in the intraday session, it closed down 24.783% the next day. As of noon on April 8, Kintor Pharmaceuticals rose 15.21% to HK$25 per share.
Prokluamide has high hopes for the first domestically produced oral drug for COVID-19. The Red Star Capital Bureau noticed that although the subject’s 100% protection rate does not mean that the patient can be cured 100%, Fosun Pharma (600196.SH; 02196.HK) has already targeted its market before it has been approved for listing. value, obtained the exclusive registration and commercialized sales rights of Prokluamide in advance, and paid 560 million yuan for this.
On April 8, Red Star Capital Bureau contacted Kintor Pharmaceutical about the relevant trial situation, but the other party declined the interview.

Data map according to IC photo
China’s first published phase III clinical trial results
oral drug for COVID-19 p>
On April 6, Kintor Pharma announced the key data results of the Phase III clinical trial of the new coronavirus treatment drug Proludamide in the treatment of mild to moderate non-hospitalized patients with the new crown. The results showed that 100% protection rate was achieved in subjects who completed taking the drug for more than 7 days and subjects of middle and high age with high risk factors.
The announcement also pointed out that proclutamide can reduce hospitalizations, mortality, novel coronavirus load and improve related novel coronavirus symptoms.

Screenshot from Kintor Pharma announcement
As early as April 24, 2021, Kintor began to enroll the world’s first subject. On December 24, 2021, the global enrollment of 733 subjects (727 from the United States and the rest from other countries) was completed.
On December 27, 2021, Kintor announced that the interim analysis of the Phase III clinical trial of Prokalutamide did not reach statistical significance, and the purpose of the trial was to evaluate Prokalutamide Efficacy and safety in the treatment of non-hospitalized COVID-19 patients. The Company will seek consent from various regulatory agencies, including the U.S. Food and Drug Administration (FDA), to revise the clinical trial protocol, and plans to continue recruiting high-risk COVID-19 patients with underlying diseases and no history of vaccination against COVID-19.
The next day, Kintor held an emergency conference call before the market opened, explaining that the clinical trial still verified the safety of Prokalutamide, but the company’s stock price still fell by more than 70% on the 28th, and its market value was Evaporated tens of billions of Hong Kong dollars.
Although Kintor said in the announcement that it would revise the clinical trial plan, according to several media reports, Kintor had responded that the recruitment of patients for clinical trials had been completed when the interim results were announced. , Considering various aspects, the company decided to complete this clinical trial according to the original plan. Kintor also stated that “the media misinterpreted the previous interim analysis results, and the previous interim analysis results did not represent clinical failure .”
until 2022 On February 3, the final visit of the last subject was completed.
In the past year, the share price of Kintor Pharmaceuticals has also experienced twists and turns with the progress of clinical trials, but it is undeniable that proclutamide is still regarded as the next step after Pfizer’s new coronavirus treatment drug nematevir tablets. After the conditional approval (February 11, 2022) of the combination package of ritonavir/ritonavir tablets (i.e. Paxlovid), the first most likely domestically produced oral drug for COVID-19.
Pruclamide was unexpectedly found to treat COVID-19
Originally used According to public information in the treatment of prostate
, Kintor Pharmaceutical was established in 2009, and was listed on the New Third Board in 2016 before landing on the Hong Kong Stock Exchange. The official website of Kintor Pharmaceuticals shows that its management team includes scientists and executives from companies such as Pfizer, Merck & Co, and WuXi Biologics.
The company is mainly committed to AR (androgen receptor) related diseases and tumors, as well as new crown, prostate cancer, breast cancer, liver cancer, alopecia and acne and other diseases that lack effective treatments and have a large population base. Cancer and other disease areas. The product line mainly includes small molecule innovative drugs, biological innovative drugs and combination therapies.
Prokluamide was found to reduce hospitalization and mortality in patients with COVID-19, which was actually an accidental discovery. Before being defined as “the first and most likely domestically produced oral drug for COVID-19”, proclutamide has significant clinical value in the treatment of prostate cancer.
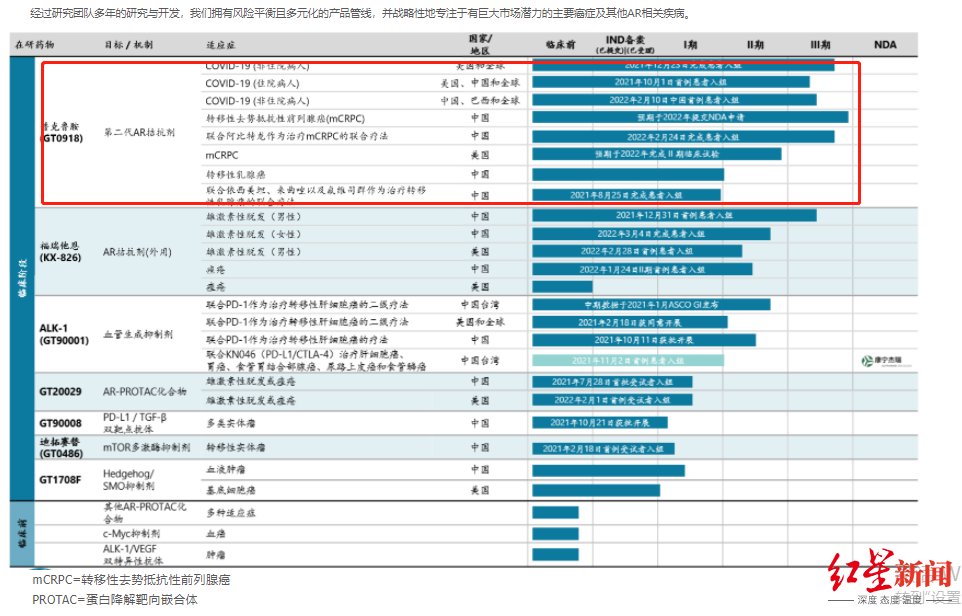
Konor Pharma’s research products including proclutamide, picture According to the official website
Prokluamide has not been approved for marketing
Fosun Pharmaceuticals have already made arrangements in advance
Although Prolutamide has not yet been approved for listing in China, Fosun Pharma (600196.SH) has already smelled business opportunities and won its overseas market. Sale Rights.
In July 2021, Proclutamide received an Emergency Use Authorization (EUA) in Paraguay for the treatment of hospitalized patients with COVID-19. At the same time, Kintor Pharma announced that it has cooperated with Fosun Pharma’s subsidiary – Shanghai Fosun Pharma Industry Development Co., Ltd. (hereinafter referred to as “Fosun Pharma Industry”) on the treatment of new crowns with proclutamide Commercialization in 10 countries reached a cooperation agreement.
Fosun Pharmaceutical Industry will obtain the exclusive registration and commercial sales rights of Prokluamide in the cooperation area, and Kintor Pharmaceutical can receive a down payment of no more than 560 million yuan.
The company’s annual report shows that the company is still in a state of loss, with losses of 233 million yuan, 508 million yuan and 842 million yuan respectively from 2019 to 2021, with a cumulative loss of about 1.583 billion yuan.
According to the accumulated losses in the past three years, the exclusive registration and sales expenses paid by Fosun Pharma can basically cover the company’s losses for one year.
It is worth mentioning that judging from its latest 2021 annual report, the company’s R&D costs increased from 329 million yuan in 2020 to 768 million yuan in 2021, a growth rate of 133.5%, mainly due to the fact that the group initiated the report during the reporting period. and three trials of proclutamide for the treatment of COVID-19.
In addition to the export, Kintor also introduced it. In January 2018, the company announced that Pfizer would grant Kintor the exclusive right to develop, manufacture and commercialize a new anti-tumor antibody drug worldwide. This is the first time that Pfizer has authorized a local Chinese company to develop the right to develop new antibody drugs in the field of oncology. The drug is also expected to become the world’s first fully human monoclonal antibody therapeutic drug targeting Activin receptor-like kinase-1 (ALK-1).
Explaining doubts: 100% protection rate
It does not mean 100% cure, but control Effect
However, proclutamide did not bring a firm expectation to the market.
A careful study of the announcement shows that the participants in the above-mentioned Phase III clinical trial are “non-hospitalized patients with mild to moderate symptoms”. The company’s other ongoing Phase III clinical trial (NCT04869228) for non-hospitalized patients with mild to moderate illness has been modified, and the results of the trial to expand to include high-risk groups are not yet known.
In fact, the 100% protection rate of subjects who took proclutamide for more than 7 days does not mean 100% cure, but more of a control effect.
(Note: The protection rate refers to the ratio of the difference between the incidence (or death) rate of the control group and the experimental group to the incidence (or death) rate of the control group. (or death). It is usually expressed as a percentage (%).)
A pharmacy researcher from Sichuan University explained to the Red Star Capital Bureau what a 100% protection rate is,
> “Compared with the new crown patients who did not take Prokluamide, the patients who took it did not have hospitalization or death.” “There is no hospitalization or death, although it does not mean a cure, but it can control the disease. If it does not worsen, it can also show that the drug is effective against the new crown virus.”
Compared with the oral drug, there is no clear protection rate index, the WHO believes that the protection rate of the new crown vaccine is in More than 50% can be approved for market use, which can form an effective immune barrier in the population and block the spread of infectious diseases. But even so, the WHO recommends oral medication for COVID-19 patients.
On March 4, the reference news quoted Agence France-Presse as reporting that the oral drug monapilavir developed by Merck Pharmaceuticals in the United States should be taken as soon as possible after symptoms of new coronary pneumonia appear, and it will be taken for 5 days. Can reduce the risk of hospitalization. A WHO panel of experts said in the British Medical Journal that it is recommended that people with weakened immune systems or chronic diseases take the drug orally when they have non-severe new coronary pneumonia.
But the report also said there was not much evidence that the drug would reduce the death rate, with only 6 fewer deaths per 1,000 patients.
Red Star News reporter Deng Lingyao
Editor Tao Yueyang
(Download Red Star News, there are prizes for reporting materials!)
