The first batch of COVID-19 antigen detection products for self-testing batch!
On March 12, CCTV news reported that the State Food and Drug Administration issued a notice approving Nanjing Novizan, Beijing Jinwofu and Shenzhen Huada. The application for self-testing of new crown antigen products of Yuanyuan, Guangzhou Wanfu Bio, and Beijing Huaketai Bio has been changed. Since then, five new coronavirus antigen self-test products have been officially launched.
On the same day, the latest pending information released by the government affairs portal of the State Food and Drug Administration showed that the medical device approval certificates for 4 new crown antigen detection kit products were obtained. The documents (changes) are to be received, and the approval time was on March 12, involving 4 companies, including Nanjing Novizan Biotechnology Co., Ltd. (Novizan, 688105), a wholly-owned subsidiary of Nanjing Novizan Medical Technology Co., Ltd. , Guangzhou Wanfu Biotechnology Co., Ltd. (Wanfu Biological, 300482), Shenzhen Huada Yinyuan Pharmaceutical Technology Co., Ltd., a subsidiary of BGI (300676), and Beijing Jinwofu Bioengineering Technology Co., Ltd.
In addition to Beijing Huaketai, which was approved to change the day before, the number of manufacturers approved to change has increased to 5.

On March 11, the previous day, the government affairs portal of the State Food and Drug Administration The information to be received shows that the medical device approval document (change) for the new crown antigen detection product of Beijing Huaketai Biotechnology Co., Ltd. is to be received, and the approval date is March 11.
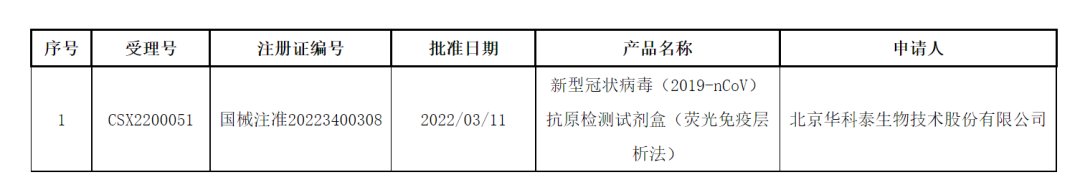
At present, 5 new crown antigen detection products from 5 companies in China have been approved. It is the above 5 products whose information has been changed. Previously, these products were only approved for professional use, not self-testing. The information about the changes to be received this time was announced. The State Food and Drug Administration did not disclose the details of the product changes. Some people in the industry believe that the information of this change may be allowed to be used for self-testing by residents.
On March 12, The Paper reporter contacted Nanjing Novizan’s service hotline. A staff member said on the phone that the official website of the Food and Drug Administration is currently Regarding the change information of the new crown antigen detection product, it is indeed changed from the professional version to the self-test version. As for whether the production of the self-test version starts, the above-mentioned staff said that it is not clear.
Also on March 11, the National Health and Health Commission issued the “New Coronavirus Antigen Detection Application Plan (Trial)”, which clearly stated that on the basis of nucleic acid detection, increase Antigen testing is supplemented. It is mentioned that if community residents have self-testing needs, they can purchase antigen testing reagents for self-testing through retail pharmacies, online sales platforms and other channels.
In just one day, five new coronavirus antigen self-test products were officially launched.
Expert Details
Nucleic acid detection is the “gold standard”, antigen detection is more convenient
Antigen testing can be used as a supplementary method for screening specific populations span>
Why add antigen detection as a supplement? What are the advantages of antigen testing? How does antigen detection work well with nucleic acid detection? The Paper interviewed Professor Lu Hongzhou, a famous infection scholar, president of Shenzhen Third People’s Hospital, and academician of the American Academy of Microbiology. span>
The Paper: Nucleic acid testing plays a pivotal role in my country’s normalized epidemic prevention and control, why this time Add an antigen test?
Lu Hongzhou: According to the latest version of the “New Coronary Virus Pneumonia Diagnosis and Treatment Plan (Trial Version 8 Revised Edition)” “, real-time fluorescence quantitative PCR detection of new coronavirus nucleic acid positive is one of the most important indicators of confirmed patients. “Early detection, early diagnosis, early isolation, and early treatment” are important strategies for the prevention and control of the new crown epidemic. After the outbreak, large-scale nucleic acid screening of close contacts and high-risk groups is an important method for early detection of infected patients.
But with the emergence of immune evasion and more infectious strains of Omicron, we can see ongoing outbreaks around the world . There have been sporadic and clustered outbreaks in many provinces and cities in my country, and the demand for nucleic acid testing in various places has shown an exponential growth trend. You can see that our nucleic acid testing has changed from the initial single test, to “5 mixed 1”, “10 mixed 1”, and now “20 mixed 1” to meet the growing demand for nucleic acid testing.
Compared with nucleic acid testing, antigen testing has the advantages of convenience, speed, and low cost. Ordinary people can easily perform home testing. In November 2020, the State Food and Drug Administration approved two novel coronavirus antigen detection kits for emergency approval. This is the first time my country has approved a novel coronavirus antigen detection kit, and the product detection time is within 20 minutes. Based on the advantages of antigen detection, the use of antigen detection kits for self-test can help us quickly find some infected people, and can also reduce the possible exposure risk during the nucleic acid sampling process to a certain extent.Acid detection provides a good complement. At present, Europe, the United States, Australia, Singapore, and Japan have all started prevention and control strategies centered on antigen detection.
The Paper: The above-mentioned “Plan” mentioned that the monitoring mode of “antigen screening and nucleic acid diagnosis” will be promoted. So, what are the characteristics of nucleic acid detection and antigen detection, and how to cooperate well?
Lu Hongzhou: The current nucleic acid detection methods are real-time quantitative PCR, which has the characteristics of high sensitivity, Studies have shown that the sensitivity of most real-time PCR assays is above 10³ copies per milliliter, so nucleic acids in samples can also be detected in the case of low gene copy numbers.
Compared with nucleic acid detection, the sensitivity of antigen detection is much lower, and the research results show that it is about 10⁵ copies per milliliter. For example, some studies have evaluated the consistency of antigen detection and nucleic acid detection, and found that when the Ct value (Editor’s Note: an indicator of laboratory testing, the lower the value, the higher the viral load of the patient) is less than 25, antigen detection and nucleic acid detection. The consistent performance of detection reaches 95.8%; when it is higher than 25, the consistency is only about 50.7%; when the Ct value is higher than 30, it is only about 20.9%.
Nevertheless, the presence of viral nucleic acid does not mean the presence of infectious live virus. Studies have found that many RNA (ribonucleic acid) positive samples do not. Infectious, especially in samples longer than 14 days. In contrast, based on the characteristics of antigen detection, some studies have found that the use of antigen detection to detect infectious viruses in patients has high sensitivity.
Therefore, based on the dynamics of the virus in the human body, the researchers summarized the efficacy of antigen detection and nucleic acid detection to find infectious patients, as shown in the figure below (The picture shows the interviewee’s modification from an article in the internationally renowned medical journal “British Medical Journal”; Alex Crozier et al. BMJ 2021;372:n208).
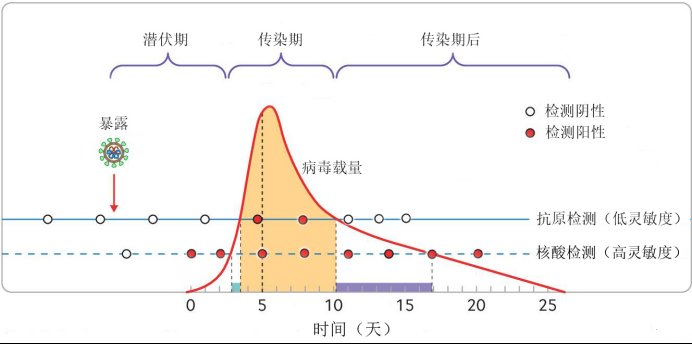
Efficacy comparison of antigen detection and nucleic acid detection for infectious patients. Photo provided by the interviewee
We can see that nucleic acid detection can detect infected patients very early, and then have a very accurate monitoring of the entire detoxification cycle of patients. Although antigen detection has low sensitivity and cannot detect patients after the incubation period and infectious period, its sensitivity can cover the entire infectious period. Therefore, the vast majority of infectious cases can be detected by multiple antigen tests (such as 3 times a week or more). In this way, antigen testing can provide a good supplement to nucleic acid testing for grass-roots medical personnel, isolation and observation personnel, or other personnel who need self-examination.
The Paper: The above-mentioned “Plan” proposes that residents can purchase antigen detection reagents for self-testing through retail pharmacies, online sales platforms and other channels. . So do ordinary residents still need to go for nucleic acid testing?
Lu Hongzhou: At present, my country still implements the “dynamic clearing” prevention and control policy. We can see that although the country has just issued the “application plan (trial)” for the new crown antigen test, the antigen test is still only a supplement to the nucleic acid test. Antigen testing is mainly aimed at grassroots patients with fever symptoms, isolation and observation personnel, and residents who need self-testing. At present, based on our “dynamic clearing” epidemic prevention policy, if residents feel exposed and at risk of infection, they should go to relevant institutions for nucleic acid testing in a timely manner when conditions permit.
It should be noted that, limited by the sensitivity of antigen detection test, under my country’s current epidemic prevention policy, it is not suitable to use antigen self-test results as Evidence for medical treatment, travel, and access to public places.
The Paper: How can antigen detection play a better role in the future?
Lu Hongzhou: In the future, with the promotion of antigen testing and the update of corresponding supporting facilities, provinces and cities can also The establishment of an antigen self-test upload system similar to nucleic acid testing is complementary to nucleic acid testing, and diverts the nucleic acid testing needs of some low-risk groups, which can greatly relieve the pressure of nucleic acid testing.
Source: Comprehensive Senior Reporter Li Jiawei< /p>