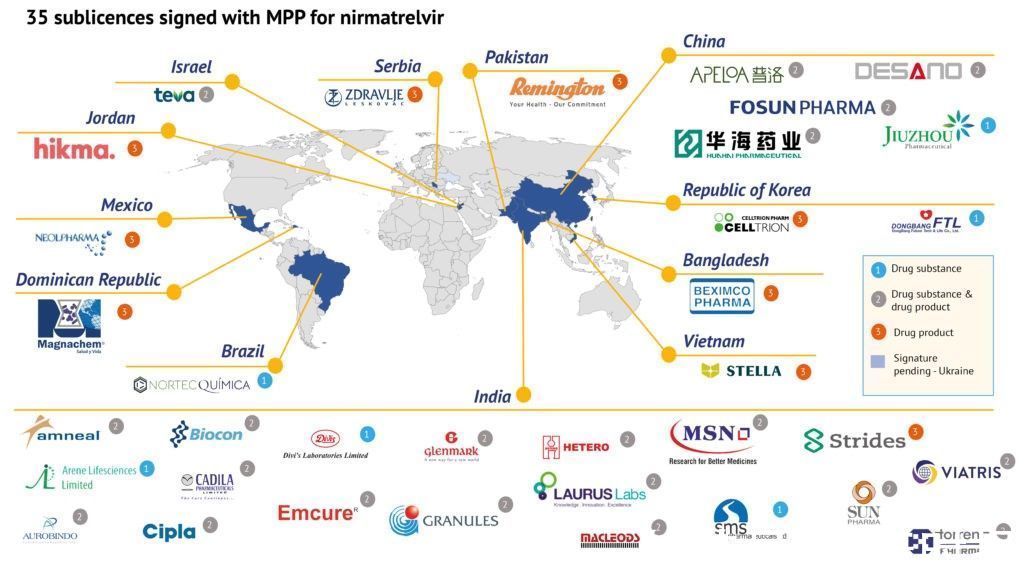
(Compiled by Health Times reporter Gao Ruirui) On March 18 (March 17 local time), the official website of the Geneva Medicines Patent Pool (MPP) announced that it has signed agreements with 35 companies to produce Pfizer oral products. A generic version of the covid-19 treatment nemaprevir, which is available in combination with low-dose ritonavir, is available in 95 low- and middle-income countries.

Source: MPP official website
According to the official website, the Drug Patent Pool (MPP) is a A United Nations-backed public health organization dedicated to increasing access and development of life-saving medicines in low- and middle-income countries. To date, MPP has signed 13 HIV antiretroviral drugs, 1 HIV technology platform, 3 hepatitis C direct-acting antiviral drugs, 1 tuberculosis treatment, two long-term A protocol for efficacy techniques, 2 experimental oral antiviral treatments for covid-19, and a covid-19 serological antibody diagnostic test.
Source: MPP official website
Health Times reporter noticed that there are 5 medicines in China this time The company is authorized to imitate the production of Pfizer’s new crown oral drugs, namely Fosun Pharma, Huahai Pharmaceutical, Puluo Pharmaceutical, Jiuzhou Pharmaceutical, and Shanghai Desano. Among them, Jiuzhou Pharmaceutical only produces raw materials, and the rest can produce raw materials at the same time Medicines and preparations.
These sublicensing agreements are understood to be the result of a voluntary licensing agreement signed by MPP and Pfizer in November 2021 that will facilitate the supply of these drugs to countries home to approximately 53% of the world’s population . These companies cover 12 countries: Bangladesh, Brazil, China, Dominican Republic, Jordan, India, Israel, Mexico, Pakistan, Serbia, Republic of Korea and Vietnam. A Ukrainian company was also granted a license, but they were unable to sign due to the current conflict, and they can still get a license.
While Pfizer negotiated an agreement with MPP establishing terms and conditions, MPP reviewed and submitted to Pfizer the off-patent producer’s sublicense request. As covid-19 remains a public health emergency of international concern by the World Health Organization, Pfizer will not receive royalties from the sales of nimatrevir by MPP sublicensors. After the pandemic period, sales to low-income countries will remain royalty-free, and low- and upper-middle-income countries will enjoy a 5% royalty on sales to the public sector and a 10% royalty on sales to the private sector.
Pfizer Chairman and Chief Executive Officer Albert Brah said: “We have established a comprehensive strategy with global governments, international global health leaders and global manufacturers to help patients in need around the world. Access to oral covid-19 treatment. The additional capacity of MPP’s sublicensors and the covid-19 treatment they will provide will play a key role in helping to ensure that people around the world, especially those living in the world’s poorest regions, have access to equity access to oral treatment options for Covid-19.”
References:
- 35 generic manufacturers sign agreements with MPP to produce low-cost, generic versions of Pfizer’s oral COVID-19 treatment nirmatrelvir in combination with ritonavir for supply in 95 low- and middle-income countries.