Recently, the State Food and Drug Administration issued an announcement that potassium glucosamine sulfate tablets were converted from prescription drugs to non-prescription drugs. The announcement requires that the relevant drug marketing authorization holders should submit the revised instructions to the provincial drug supervision and administration department for record before April 6, 2023, in accordance with the “Drug Registration Management Measures” and other relevant regulations, and notify the relevant medical personnel of the revised instructions in a timely manner. Institutions, pharmaceutical companies and other units.
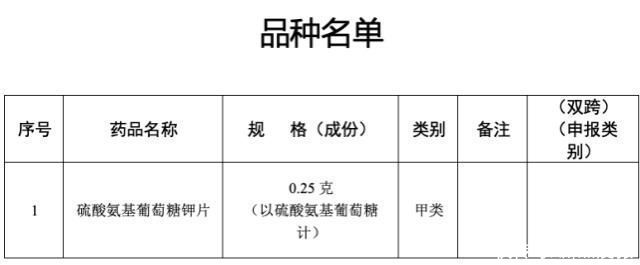
Potassium Glucosamine Sulfate Tablets are exclusive varieties of Shanxi Kangbao Biological Products Co., Ltd. This variety is an analgesic over-the-counter drug, and its indications are osteoarthritis of various joints in the body, such as knee, hip, spine, shoulder, hand, wrist and ankle.
After the potassium glucosamine sulfate is converted to an over-the-counter drug, a prescription from a licensed physician or a licensed physician assistant is not required, and patients can judge, purchase and use the drug by themselves. However, the newly released instructions show that the drug is contraindicated in three groups of people, including pregnant and lactating women, patients allergic to glucosamine sulfate or any excipients of this product, and patients with hyperkalemia.
Xiaoxiang Morning News reporter Li Shu
News clues reveal channel: Download the “Morning Video” client from the app market and enter the topic of “Morning Help”; or call the morning video news hotline 0731- 85571188.