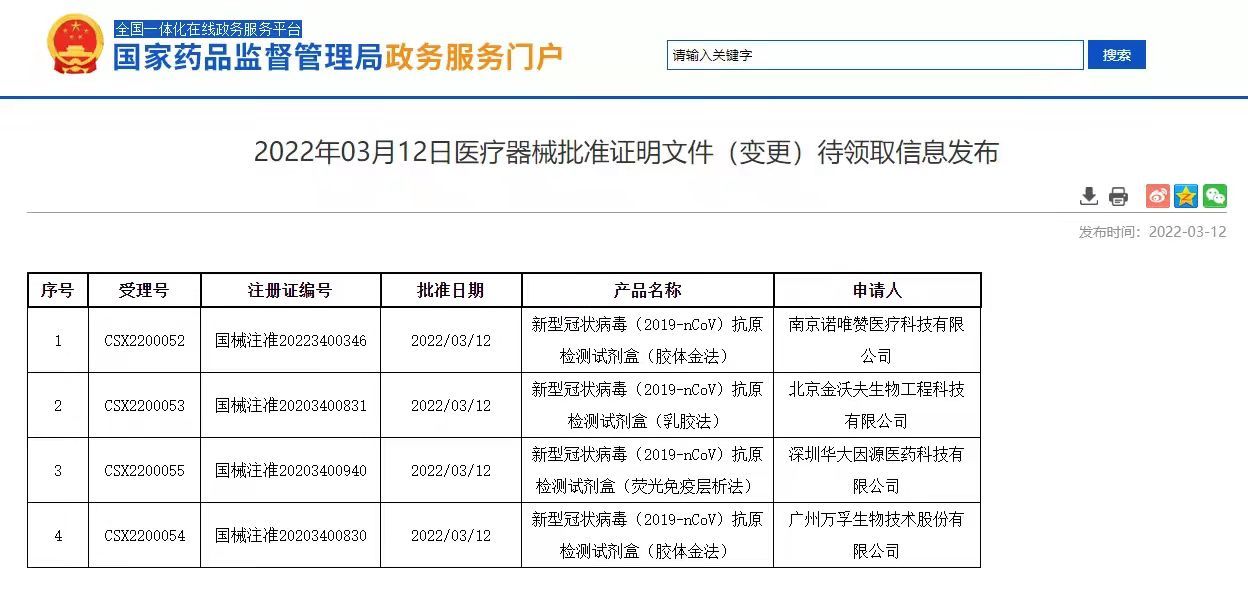
The State Food and Drug Administration issued a notice today, approving Nanjing Novizan, Beijing Jinwofu, Shenzhen Huada Yinyuan, Guangzhou Wanfu The application for self-testing of new crown antigen products of Biotech and Beijing Huaketai Biotech has been changed. Since then, five new coronavirus antigen self-test products have been officially launched.
According to the website of the National Health Commission on the 11th, in order to further optimize the new coronavirus detection strategy, the comprehensive group of the joint prevention and control mechanism of the State Council decided to add antigen detection as a supplement on the basis of nucleic acid detection. New Coronavirus Antigen Detection Application Scheme (Trial)”.
The plan specifies the applicable population for antigen testing: first, those who go to primary medical and health institutions for treatment, with symptoms such as respiratory tract, fever, and symptoms within 5 days; second, those who are quarantined and observed, including those at home Persons in isolation observation, close contact and sub-close contact, entry isolation observation, closed control area and control area; the third is community residents who need antigen self-testing.
The plan also clarifies the main conditions to be met for antigen testing for the above three groups of people, the channels for obtaining testing reagents, and formulates the disposal management process after positive testing is found, so as to promote the connection between antigen testing and nucleic acid testing.
According to the plan, community residents who have self-test needs can purchase antigen test reagents for self-test through retail pharmacies, online sales platforms and other channels.
The National Health and Health Commission also specifically stated that nucleic acid testing is still the basis for the diagnosis of new coronavirus infection, and antigen testing can be used as a supplementary method to screen specific groups of people, which is conducive to improving the ability of “early detection”.
According to the plan, primary medical and health institutions with nucleic acid detection capabilities should prefer nucleic acid detection; those without nucleic acid detection capabilities may conduct antigen testing. Isolation observers and community residents should carefully read the instructions and standardize their operations for antigen testing. Once the antigen test is positive, they should immediately report to the relevant departments; when necessary, nucleic acid testing should be carried out to confirm.
It is understood that compared with nucleic acid detection, antigen detection can be faster and more convenient to operate, but the accuracy is low, and it is generally used for suspected people in the acute infection stage. Antigen-positive results can be used for early triage and rapid management of suspected populations, but not as a basis for diagnosing new coronavirus infection.
Edit: Wang Xing
Editor in charge: Fan Liping and Gu Jun
Comprehensive: Xinhua News Agency, Xinhuanet< /p>