內容目錄
Concurrent media reporter Wang Lina and Liu Zebo
Concurrent chemoradiotherapy is the standard curative treatment for locally advanced non-small cell lung cancer (LA-NSCLC), but the efficacy is limited. Tumor recurrence within a year. In the past, imaging changes alone could not predict the prognosis of LA-NSCLC after radiotherapy and chemotherapy. How to accurately identify high-risk groups of lung cancer recurrence in order to further optimize the treatment plan has always been a problem for clinicians. So in addition to judging recurrence by imaging, can we see recurrence by “dropping blood”?

Screenshot of the article
Recently, the team of internationally renowned lung cancer radiotherapy experts Wang Luhua and Bi Nan The designed and carried out research results of circulating tumor DNA (ctDNA) predicting the prognosis of LA-NSCLC patients were published in “Molecular Cancer” (IF=27.401). This study is the first high-quality cohort study in Asia after the Stanford University team.
The study revealed that cancer recurrence in lung cancer patients can be predicted by detecting the blood of patients after radiotherapy. In 2021, the preliminary results of the study were invited to give an oral presentation at the annual meeting of the American Society of Radiation Oncology. Through dynamic sampling analysis at multiple time points, the study found for the first time that 1 month after radiotherapy was the best time for blood collection, and the ctDNA status at this time point had the best predictive ability for the prognosis of patients, which was better than the baseline level before radiotherapy and 4 weeks during radiotherapy. (Molecular Cancer, online May 19)
Study Highlights
- Based on a prospective study design, the results of Characteristics of ctDNA dynamic changes.
- The patients’ peripheral blood was collected at 5 different time points to analyze the correlation between the dynamic changes of ctDNA and prognosis, and the optimal time point for dynamic monitoring of ctDNA was clarified.
- It further expands the clinical application of ctDNA, which can be used to evaluate the prognosis of patients with locally advanced NSCLC after radical chemoradiotherapy, and then adjust the treatment plan in time.
Results
The study prospectively enrolled 55 patients with non-small cell lung cancer who were diagnosed and treated in the Department of Radiotherapy, Cancer Hospital, Chinese Academy of Medical Sciences. Peripheral blood samples were collected at different time points (baseline, week 4 during radiotherapy, 1 month after radiotherapy, 3 months after radiotherapy, and at the time of imaging progression), and a panel containing 474 lung cancer radiotherapy genes was uniformly used for ctDNA detection.
Baseline ctDNA was not a good predictor of patient prognosis
The study suggested that the baseline ctDNA level of lung cancer patients increased significantly with the increase of TNM stage, and was closely related to disease stage, tumor size and TNM stage. There was a positive correlation with lymph node metastasis. Patients were divided into ctDNA-positive and ctDNA-negative groups according to their baseline ctDNA status, but there was no significant difference in progression-free survival (PFS) and overall survival (OS) between ctDNA-negative patients and positive patients (Figure 1E, 1F), suggesting that the baseline ctDNA cannot be a prognostic marker in patients with locally advanced non-small cell lung cancer receiving chemoradiotherapy.
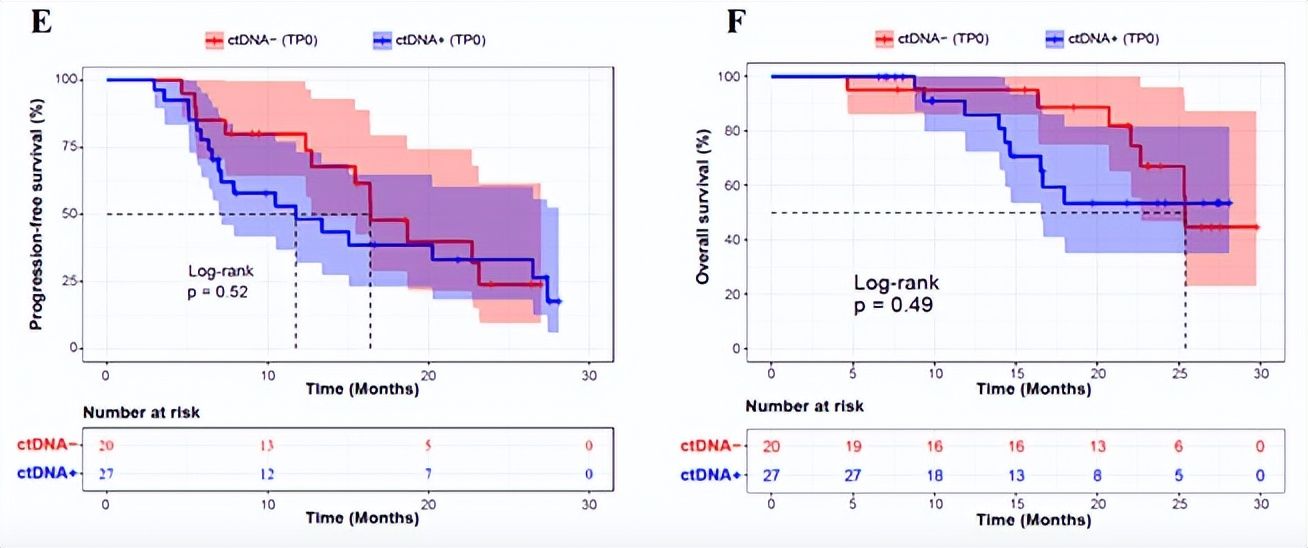
Figure 1 (E, F). Kaplan-Meier curves of PFS (A) and OS (B) of patients grouped by baseline ctDNA status< /p>
One month after radiotherapy, blood was collected to measure ctDNA, which predicted the best prognosis of patients.
The researchers performed dynamic monitoring of ctDNA at 4 time points after the start of treatment. The results showed that ctDNA at these four time points could predict the prognosis of patients to a certain extent, and the ctDNA at 1 month after radiotherapy (TP2) had the best prognostic stratification ability. The PFS and OS of ctDNA-negative patients at TP2 time point were significantly better than those of ctDNA-positive patients at this time point (P<0.0001; P=0.0016) (Fig. 2A,B). Because the unique biological characteristics of patients with brain metastases may affect the accuracy of the results, the study further analyzed the hazard ratio of the two groups of patients with brain metastases not excluded/excluded. The results still showed the best predictive power at the TP2 time point (Fig. 2C).
As a representative case, Figure 2D shows the imaging results of a patient with stage IIIA lung squamous cell carcinoma who had positive ctDNA at baseline at initial diagnosis and undetectable ctDNA at TP2, corresponding time points The tumor also shrunk significantly. The last follow-up time was 28 months from the date of diagnosis of lung squamous cell carcinoma in this patient, and the imaging data at this time point showed that the patient had no signs of tumor progression.
Meanwhile, the researchers validated this result with a test set of 20 patients with stage III non-small cell lung cancer (Figure 2E). All of the above results indicate that 1 month after chemoradiation is the best time point for collecting plasma samples to predict patient prognosis.
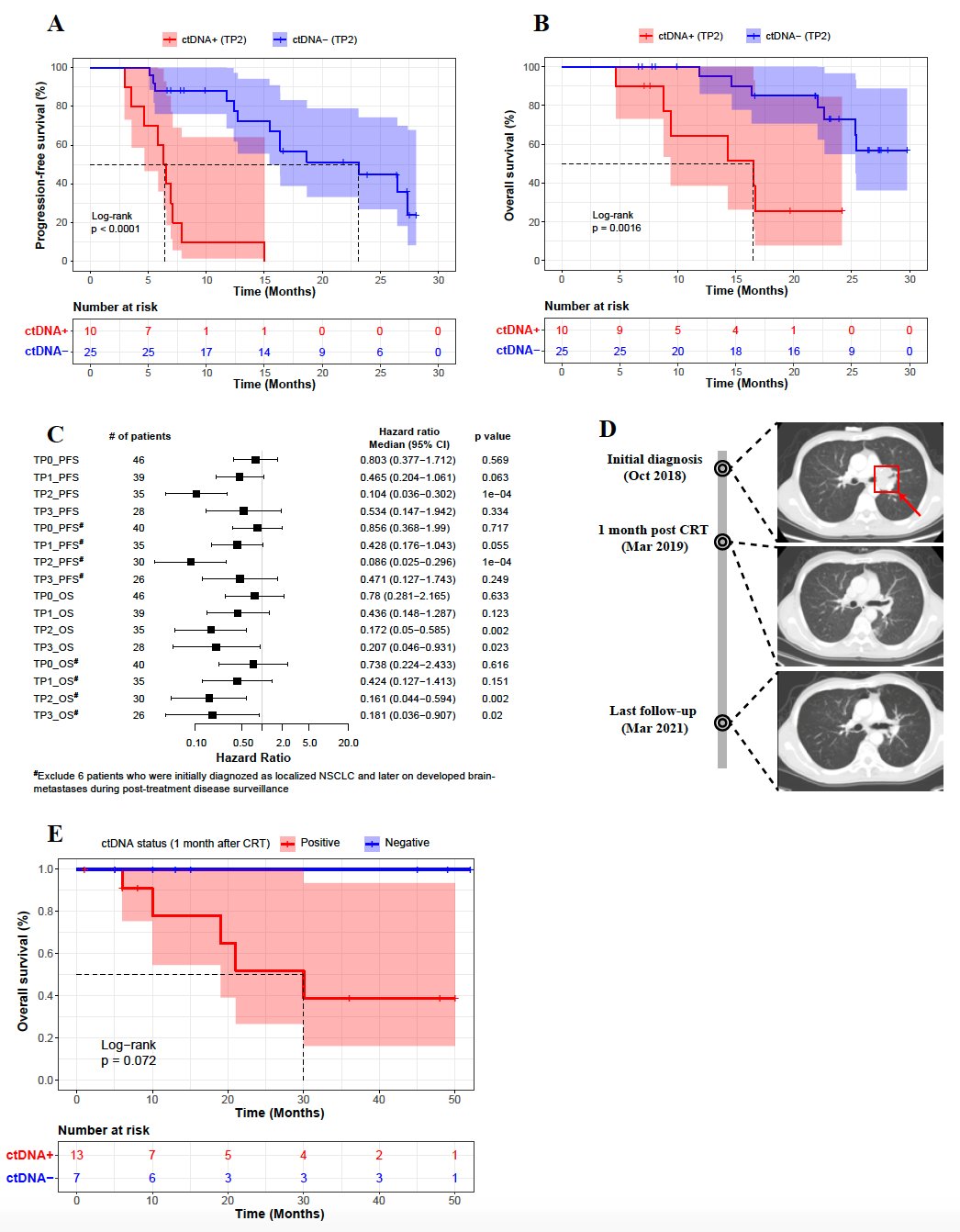
Figure 2 (A-D). Correlation analysis of ctDNA status and prognosis 1 month after radiotherapy (A-C); representative Case (D); test the correlation analysis of ctDNA and prognosis at 1 month after centralized chemoradiation (E)
The dynamic change of ctDNA has prognostic value
According to ctDNA before tumor progression Dynamic changes at different detection time points, the researchers divided patients who had blood collection at ≥3 time points into 4 groups:
Group 0 (ctDNA negative group): ctDNA detection at all time points was negative;
Group 0 (ctDNA negative group): p>
Group 1 (ctDNA reduction and clearing group): the baseline ctDNA test was positive, the dynamic monitoring ctDNA level was decreased, and the ctDNA test in the last blood collection was negative;
Group 2 (ctDNA reduction non-zero clearing group) ): The baseline ctDNA test was positive, and the dynamic monitoring ctDNA level decreased, but the ctDNA test in the last blood collection was still positive;
Group 3 (ctDNA elevated group): ctDNA level increased.
The results showed that patients in groups 0 and 1 had significantly better PFS and OS than patients in groups 2 and 3 (Fig. 3A, B).
According to whether the ctDNA test was negative in the last blood sampling before tumor progression, the researchers further divided the patients into a ctDNA-cleared group and a ctDNA-uncleared group. PFS and OS (Figure 3C,D). These findings suggest that ctDNA dynamic monitoring has the potential to predict the prognosis of locally advanced non-small cell lung cancer.
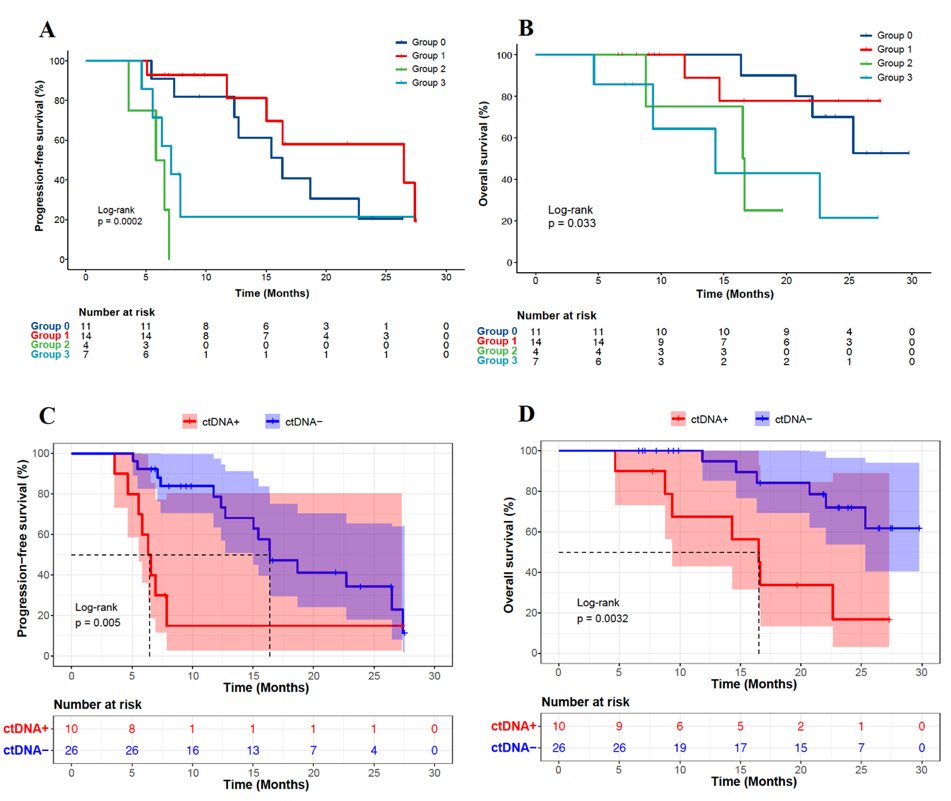
Figure 3. Kaplan-Meier curves of PFS (A) and OS (B) of patients grouped based on 4 dynamic change patterns of ctDNA, and Kaplan-Meier curves of PFS (C) and OS (D) of patients based on whether ctDNA was cleared or not
< p>Researchers say

Professor Bi Nan, Deputy Director, Department of Radiotherapy, Cancer Hospital, Chinese Academy of Medical Sciences
“Physician’s Daily”: What are the difficulties in predicting tumor recurrence in radiotherapy patients? Can imaging changes be used instead of ctDNA detection?
Professor Bi Nan: Due to the characteristics of the mechanism of radiation therapy, Imaging changes after radiotherapy have limited predictive value for efficacy and cannot replace ctDNA to predict patient prognosis. Most patients will continue to shrink their tumors 1-3 months after radiotherapy, or even half a year, and local inflammation and fibrosis will appear after radiotherapy. Benign imaging changes such as changes will also affect the judgment of CT efficacy. The results of many studies have shown that the short-term efficacy evaluated according to the RECIST criteria on CT cannot well predict the OS and long-term efficacy of LA-NSCLC after radiotherapy and chemotherapy. There is a significant difference between the radiotherapy and the simple drug treatment.
In the data analysis of this study, the correlation between the imaging changes and prognosis in one month after radiotherapy was also analyzed, and it was found that the predictive value of the imaging changes was limited and could not be better Predict the prognosis of patients.
“Physician’s Daily”: Studies have also shown that ctDNA is negative in more than 50% of patients with tumor recurrence, and its sensitivity and specificity are limited. How can this study avoid its limitations?< /h1>
Professor Bi Nan: (1) The panel used in this study contains 474 common lung cancer genes, and the sequencing depth is conventional 5000X. The detection rate is lower for patients with earlier stages. Therefore, I included in this study. -There are fewer patients with stage II, and 87% of the patients are locally advanced.
(2) All patients were collected baseline blood (collected before radiotherapy and chemotherapy at the initial diagnosis) for comparative analysis, and the baseline ctDNA was For negative lung cancer patients, the probability of positive detection by dynamic sampling during subsequent treatment and at recurrence is low, and the predictive value of ctDNA dynamic monitoring is limited. Therefore, this study will focus on patients with positive ctDNA at baseline during dynamic follow-up.
(3) Technological advances such as increasing sequencing depth are expected to improve the sensitivity of ctDNA and reduce the false negative rate.
“Physician’s Daily”: Will the positive ctDNA result appear earlier than the patient’s imaging recurrence?
Professor Bi Nan: In this study, the time point of blood collection was limited, and no systematic analysis was carried out on this issue. However, for patients with poor curative effect and early recurrence, this study did observe their ctDNA after treatment. The percentage of positives is high. In the next step, we will consider increasing the post-treatment sampling time point to provide enough data to explore this question.

Professor Wang Luhua, President of Shenzhen Hospital, Cancer Hospital, Chinese Academy of Medical Sciences
“Physician Daily”: What interventions are available for patients with poor prognosis indicated by ctDNA testing?
Professor Wang Luhua: At present, immunoconsolidation therapy after chemoradiotherapy (PACIFIC mode) has become the standard treatment for inoperable LA-NSCLC, but traditional concurrent chemoradiotherapy can cure some patients, and the results of ctDNA test are helpful. It is necessary to find these patients with good prognosis; for patients with positive ctDNA after radiotherapy with poor prognosis, it may be necessary to add immune checkpoint inhibitor therapy, consolidation therapy or targeted drugs according to the molecular characteristics of the tumor. In addition, more regular imaging review is also more important, early detection, early treatment.
“Physician’s Daily”: What is the main difference between this study and previous studies focusing on predicting tumor progression, drug resistance information, and guiding individualized precision treatment, and what pain points have been solved in previous studies? What is the clinical application of ctDNA?
Professor Wang Luhua: There are different mechanisms between radiotherapy and drug therapy. There are few studies on the prognostic value of ctDNA in patients receiving radiotherapy. This study is the first high-quality cohort study in Asia following the team from Stanford University in the United States. The results suggest that ctDNA has the best predictive ability for efficacy at 1 month after radiotherapy. If it can be applied to the clinic in the future, the testing process can be simplified, the testing cost can be reduced, and it will make an important contribution to its popularization in clinical practice. associated serious toxicities.
“Physician’s Daily”: Can the conclusions of this study be applied clinically, and in what ways will it change the clinical practice?
Professor Wang Lvhua: The research conclusions of this study have reached consistent conclusions in both internal and external verification. Combined with other current small sample studies, as well as studies on ctDNA and MRD monitoring after NSCLC, the conclusions are comparable Similarly, ctDNA/MRD status after local treatment such as surgery and radiotherapy is the best time point for efficacy prediction. However, a prospective cohort with a larger sample size is needed for validation before widespread clinical application.
ctDNA is a non-invasive, highly accurate and predictive biomarker, which is a useful supplement to traditional tumor markers and imaging methods; in addition, ctDNA can provide molecular features of tumors such as driver Gene mutation, etc., will play a role in further precise treatment after progression, avoiding re-puncture biopsy, etc.
For patients with positive ctDNA 1 month after radiotherapy, more active consolidation treatment measures should be taken, and imaging recurrence should be closely monitored, and early detection and treatment should be performed to improve the prognosis of high-risk recurrence patients.
At the same time, the research team will further explore the predictive value of ctDNA on the efficacy of immunoconsolidation therapy on the basis of this topic, and expect to further guide the choice of immunoconsolidation therapy through ctDNA detection.