On March 12, the State Food and Drug Administration issued a notice approving the application changes for self-testing and application of new crown antigen products of Nanjing Novizan, Beijing Jinwofu, Shenzhen Huada Yinyuan, Guangzhou Wanfu Bio, and Beijing Huaketai Bio. . Since then, five new coronavirus antigen self-test products have been officially launched.
Five new crown antigen self-test products were approved
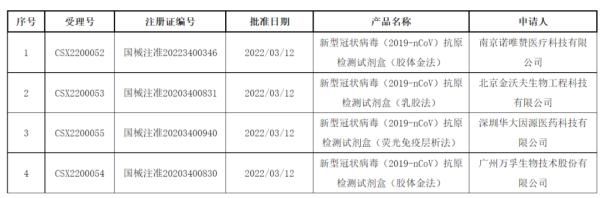
On the same day, the latest pending information released by the government affairs portal of the State Food and Drug Administration showed that four new crown antigen detection kit products were obtained. The medical device approval documents (changes) of the medical device approval documents (changes) are pending, and the approval time was on March 12, involving 4 companies, including Nanjing Novozymes, a wholly-owned subsidiary of Nanjing Novozymes Co., Ltd. Zan Medical Technology Co., Ltd., Guangzhou Wanfu Biotechnology Co., Ltd. (Wanfu Biological, 300482), Shenzhen Huada Yinyuan Pharmaceutical Technology Co., Ltd., a subsidiary of BGI (300676), and Beijing Jinwofu Bioengineering Technology Co., Ltd. In addition to Beijing Huaketai, which was approved to change the day before, the number of manufacturers approved to change has increased to five.
On March 11, the pending information released by the government affairs portal of the State Food and Drug Administration showed that Beijing Huaketai Biotechnology Co., Ltd. The medical device approval document (change) of the new crown antigen detection product of the Co., Ltd. is pending, and the approval date is March 11.

At present, 5 new crown antigen detection products of 5 companies in China have been approved, and it is the above 5 change information The product.
Previously, these products were only approved for professional use, not self-testing. The information about the changes to be received this time was announced. The State Food and Drug Administration did not disclose the details of the product changes. Some people in the industry believe that the information of this change may be allowed to be used for self-testing by residents.
On March 12, the reporter contacted the service hotline of Nanjing Novizan. A staff member said on the phone that the current information on the change of the new crown antigen detection product on the official website of the Food and Drug Administration is indeed from the professional. The version was changed to a self-test version, and the above-mentioned staff said that it was not clear whether the self-test version would start production.
National Health and Health Commission:
You can purchase reagents for self-testing of new coronavirus antigens
On March 11, the National Health and Health Commission released the “New Coronavirus Antigen Detection Application” Plan (for Trial Implementation)”, it is clear that on the basis of nucleic acid testing, antigen testing will be added as a supplement. It is mentioned that if community residents have self-testing needs, they can purchase antigen testing reagents for self-testing through retail pharmacies, online sales platforms and other channels.
In just one day, five new coronavirus antigen self-test products were officially launched.
What is an antigen test?
The detection methods of the new coronavirus include nucleic acid detection, antibody detection, antigen detection and other methods.
On March 11, Dr. Zhang Wenhong said in an interview with the media that antigens are like “clothes” worn on the outside of the virus, and nucleic acids are the genes inside the virus. Antigen detection starts from antibodies to detect “clothes”. After the specificity is integrated, the virus can be displayed; nucleic acid detection needs to be done through amplification. With amplification, the sensitivity of nucleic acid detection will be higher, but Correspondingly, it takes longer to obtain results.
It should be pointed out that antigen detection cannot completely replace nucleic acid detection.
The “Basic Requirements and Procedures for Self-Testing of New Coronavirus Antigens” issued by the National Health and Medical Commission emphasizes that antigen testing is generally used in the acute infection period, that is, sample testing within 7 days of the onset of symptoms of suspected people.
Suspected population with positive and negative antigen results should undergo further nucleic acid testing. Positive results can be used for early triage and rapid management of suspected populations, but cannot be used as the basis for the diagnosis of new coronavirus infection. Nucleic acid testing is still the basis for the diagnosis of new coronavirus infection.
Comprehensive: CCTV News, The Paper
Source: CCTV