3.2 Extranodal marginal zone lymphoma
Extranodal marginal zone lymphoma (EMZL) is a rare disease, accounting for 5%-8% of all B-cell lymphomas. EMZL can occur at any mucosal site, but most commonly involves the stomach (30%), ocular adnexa (12%), skin (10%), lungs (9%), and salivary glands (7%). EMZL is mostly diagnosed at an early stage, and the lesions can be limited to the origin for a long time. For patients with stage I EMZL of any site, including those with gastric involvement after failure of H. pylori eradication therapy, radiation therapy is the preferred approach.
However, 20%-40% of EMZL patients are advanced and have poor outcomes. Treatment of patients with advanced disease remains controversial, and in the absence of randomized clinical trial data, there is still a lack of consensus on the choice of first-line therapy and the number of treatment cycles.
For untreated patients with EMZL, explored drugs include single-agent rituximab, lenalidomide + rituximab, R-CVP (rituximab, phosphoramide, vincristine, and prednisolone), R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), and others.
IELSG, the only phase III study focused on first-line treatment of EMZL, randomly assigned 454 patients to rituximab plus chlorambucil, chlorambucil alone or 5-year event-free survival (EFS) was significantly longer in the rituximab monotherapy group (68%) than chlorambucil (51%) or rituximab (50%; P = 0.0009) single agent, and no significant toxicity was observed in any treatment group. Although chlorambucil in combination with rituximab has shown superior efficacy, this regimen has not yet gained widespread acceptance in the United States.
NCCN guidelines list bendamustine in combination with rituximab (BR) as one of the 3 preferred regimens for EMZL, but data supporting this regimen for the treatment of EMZL are limited. Two additional randomized clinical trials examined BR versus R-CHOP and/or R-CVP, but mainly included patients with follicular lymphoma and a relatively small number of patients with MZL, even though both trials included all MZL subtypes. analysis. MALT2008-01, the largest study to examine BR in untreated EMZL patients, achieved a CR/CRu rate of 98% in 57 evaluable patients receiving BR through a response-adaptive strategy, corresponding to a 7-year EFS rate of 88% , and no differences were observed in response rates or survival between disease sites or the presence or absence of t(11;18).
The number of patients in previous MZL clinical trials is small, and larger observational studies are needed to provide accurate data on the safety and efficacy of BR in the treatment of naïve EMZL. Therefore, Professor Izidore S. Lossos of Sylvester Comprehensive Cancer Center in the United States explored the efficacy of the BR regimen in 237 cases of newly treated EMZL, which was recently published in “blood advances”.
Results
A total of 237 patients were included in this analysis, including 182 EMZL patients whose data were collected retrospectively and 55 patients who were prospectively enrolled in the NF10 project and received first-line BR therapy. patient.
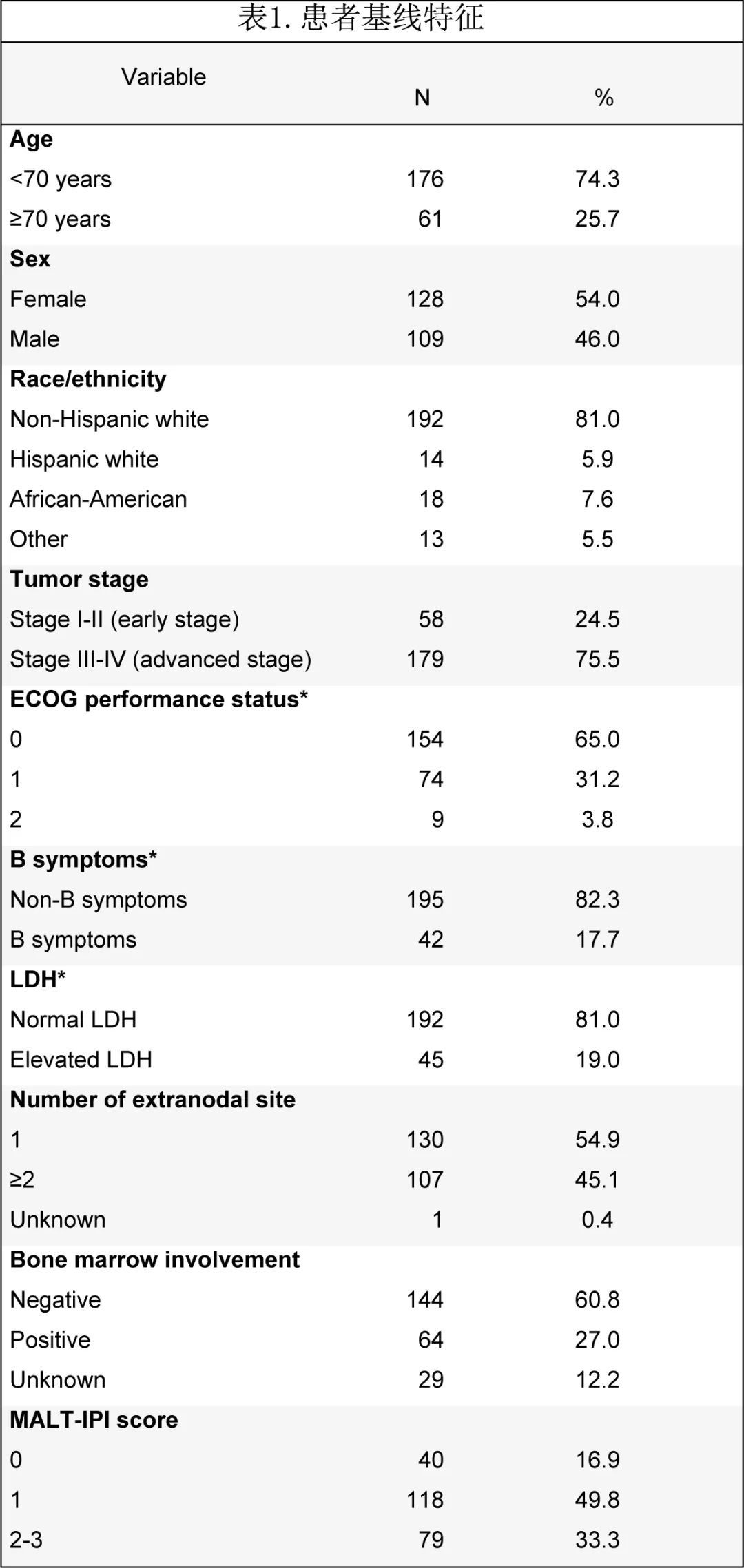
The median age at diagnosis was 63 years, and the male to female ratio was 1.2:1. Twenty-nine patients (12%) had a history of autoimmune diseases, including Hashimoto’s thyroiditis and rheumatoid arthritis ( 4 patients each) the most common. Most patients had an ECOG PS of 0-1 (65% had a PS of 0 and 31.2% had a PS of 1). Baseline staging examinations included 68 (28.7%) CT and 169 (71.3%) PET/CT. 179 (75.5%) patients were advanced (III-IV), but elevated LDH (19%, n = 45) and B symptoms (17.7%, n = 42) were uncommon. Most patients had no bone marrow involvement (n = 144, 60.8%), and most had <4 intranodal sites (79.7%, n = 189). Most patients had one extranodal lesion (54.9%, n = 130), followed by two (27.4%, n = 65) and three (12.7%, n = 30) lesions; common extranodal sites included lung (25.3%, n = 60), stomach (17.3%, n = 41) and soft tissue (10.1%, n = 24). Only 2 patients had H. pylori-associated gastric EMZL (4.4%), and both had antibiotic treatment failure before receiving BR. MALT-IPI risk was intermediate (1 risk factor) in 49.8% of patients and high risk (≥2 risk factors) in 33.3% (Table 1). Paraproteins were examined in 160 (67.5%) patients, with paraproteins present in 63 (39.4%) patients, IgM being the most common paraprotein (total 40: IgMκ 24 IgMλ 8, 8 unspecified), This was followed by IgG (5 cases each for IgGλ and κ), free light chain (4 cases), two paraproteins (2 cases) and unknown (7 cases).
Treatment data were available for 181 patients with a median time from diagnosis to BR administration of 56 days, with a median Dosing for 6 cycles; 48 patients (20.3%) received rituximab maintenance for a median of 16 months. 140 patients (59%) were evaluated for treatment response with PET/CT, and the rest underwent CT scans.
The ORR of first-line BR was 93.2%, the CR rate was 81%, and the PR rate was 12.2%. Among patients with response assessed by PET/CT, ORR was 96.5% and CR was 82.9%; among patients with response assessed by CT, ORR was 92.5% and CR was 81.7%. Patients with localized disease (stages I-II) had higher CR rates compared with patients with advanced (III-IV) disease (93% and 79%, respectively; P = 0.016).
Data from 180 patients were available, and the incidence of infectious complications during BR treatment was 13%: 7 shingles reactivation (4%), 5 pneumonia (3%), and influenza (3%) ). Among patients receiving rituximab maintenance therapy, neutropenia occurred in 3 patients (6.2%), shingles reactivation in 1 patient (2%), and facial cellulitis (2%). Eight patients (3.4%) developed secondary malignancies after BR treatment, including one each of AML and cutaneous squamous cell carcinoma (SCC).
36 patients had disease progression and 18 died. With a median follow-up of 3.21 years, the estimated 5-year PFS and OS were 80.5% and 89.6%, respectively (Figure 2A and B).
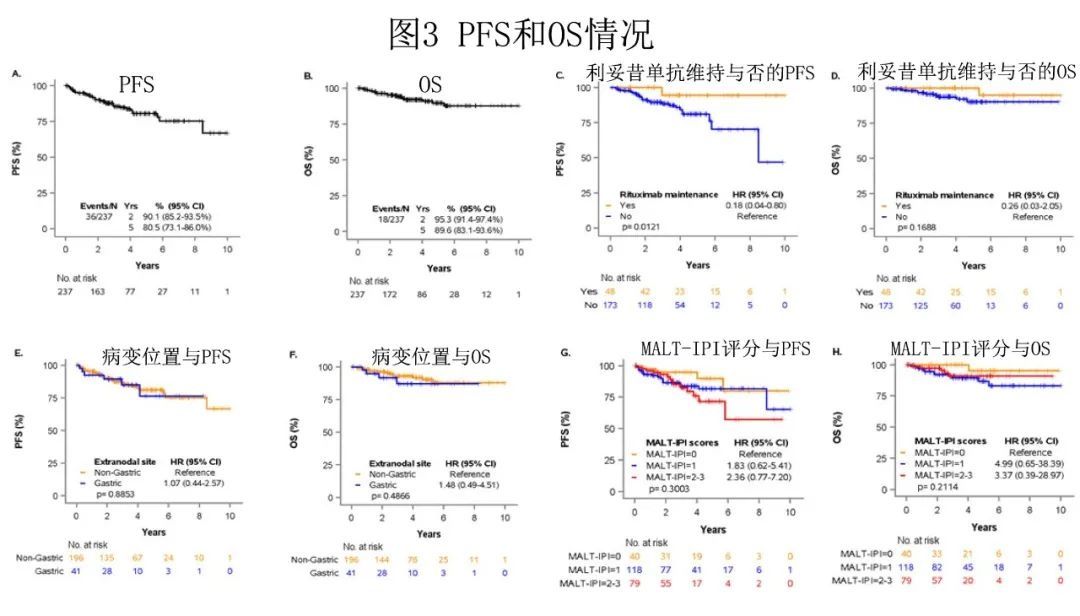
The 5-year cumulative incidence of lymphoma-specific deaths was 3.8%, with only 8 deaths attributed to lymphoma . No difference in PFS was observed among patients who achieved CR or PR after BR (HR = 0.47, 95% CI 0.18 – 1.27; P = 0.12), and the number of BR cycles was not associated with better PFS (P = 0.222; but Patients who received 1-3 cycles of therapy had shorter OS (P = 0.009). Patients who achieved CR after BR or received rituximab maintenance therapy after PR had longer PFS (5-year incidence 94.4% vs 81.1%; P= 0.012), but there was no difference in OS (P = 0.17) (Figure 2C and D).
There was no statistically significant difference in PFS or OS between the stomach and other EMZL locations (PFS P = 0.89 and OS P = 0.49) (Figure 2E and F). And interestingly, MALT-IPI scores did not predict PFS and OS (Fig. 2G and H). Furthermore, due to the low number of early progression events (n = 21), the study was unable to estimate the effect of disease progression over 24 months (POD24) on patient outcomes.
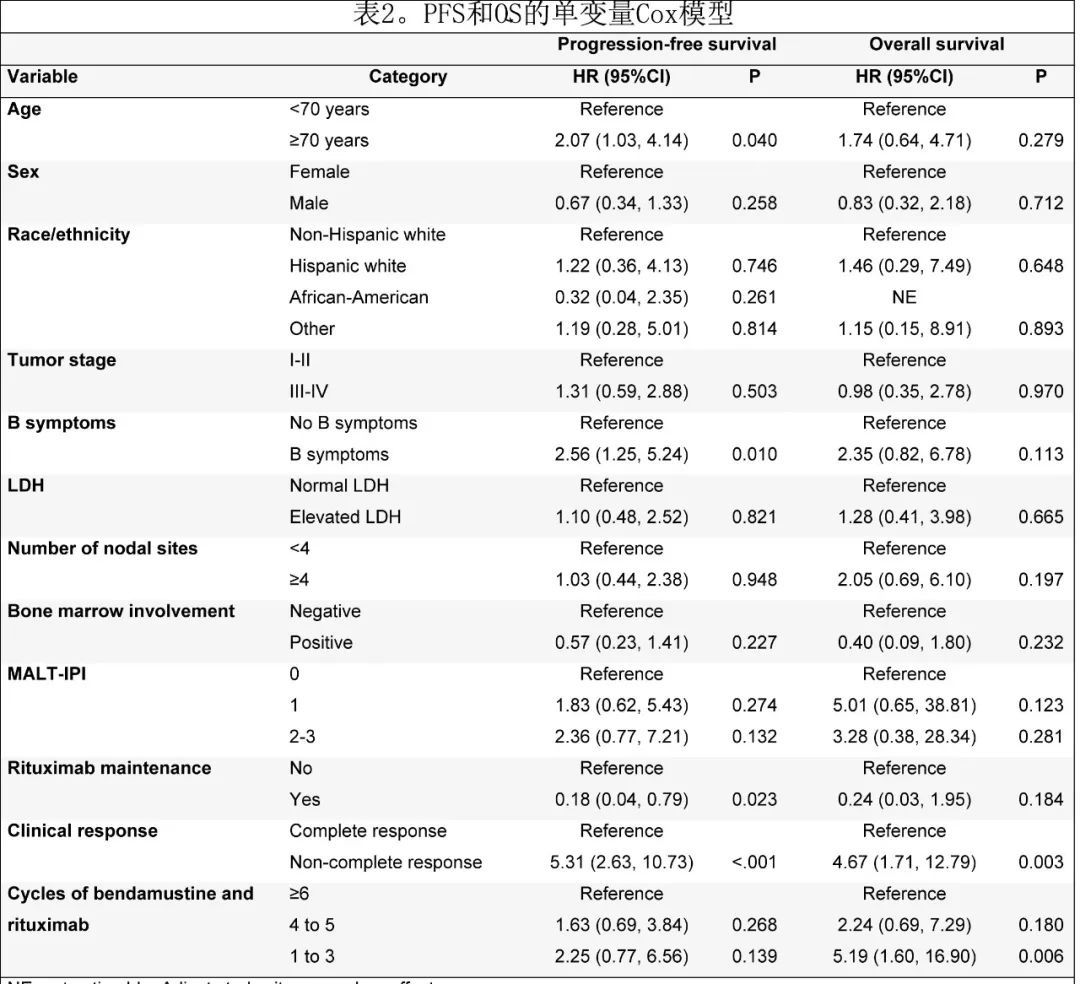
In univariate Cox regression analysis, variables associated with shorter PFS were age ≥70 years (HR = 2.07, 95%CI 1.03-4.14; P = 0.040), B symptoms (HR = 2.56) , 95%CI 1.25-5.24; P = 0.01) and lack of CR (HR = 5.31, 95%CI 2.63-10.73; P < 0.001), while rituximab maintenance (HR = 0.18, 95%CI 0.04) -0.79; P = 0.023) was associated with longer PFS. Variables associated with shorter OS were not achieving CR (HR = 4.67, 95%CI 1.71 to 12.79; P = 0.003) and receiving 1-3 cycles of BR (HR = 5.19, 95%CI 1.60 to 16.90; P = 0.006) (Table 2).
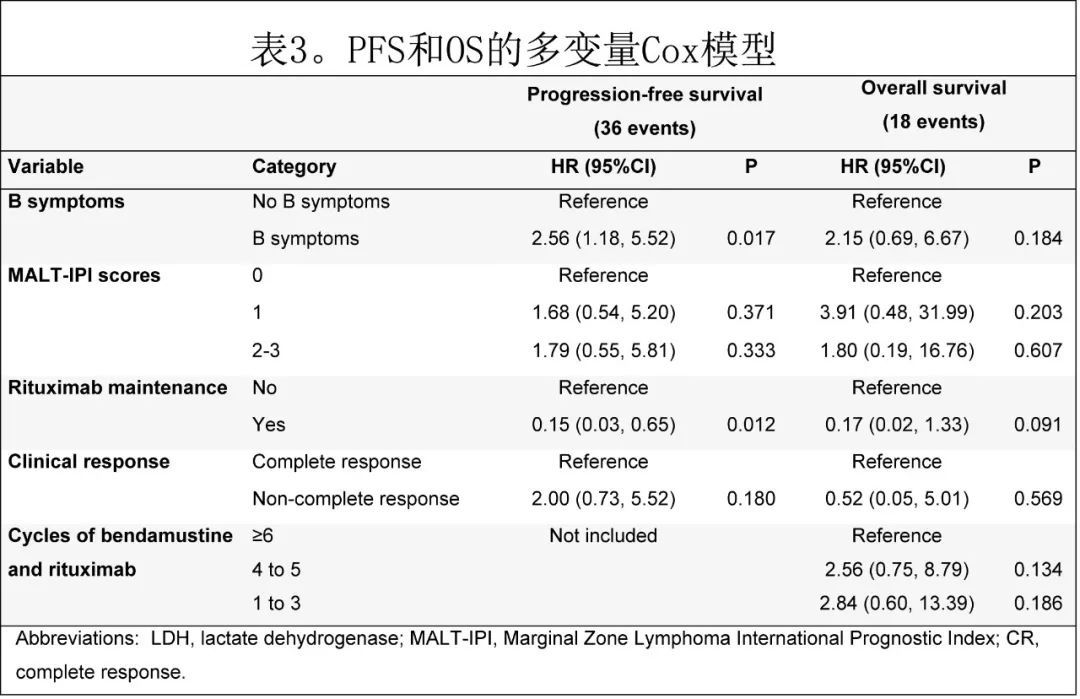
In the multivariate Cox model, the presence of B symptoms and the absence of maintenance rituximab were associated with significantly shorter PFS correlated, but no variable was associated with OS, possibly due to the low number of events (Table 3).
High-grade transformation: During follow-up, 11 patients (4.6%) developed biopsy-proven high-grade transformation (Higher grade transformation, HGT) was diffuse large B-cell lymphoma, most of which showed non-germinal center type (55.6%). The 5- and 10-year cumulative incidence of HGT with death as a competing risk was 6% and 10%, respectively (Figure 3).

The median time to HGT was 0.74 years. Patients with HGT had a higher median SUV on diagnostic PET/CT than those without transformation (18 vs 6.7, respectively; P = 0.016); patients with subsequent HGT had a median number of BR cycles of 6, but only HGT occurred in 1 (9%) patient receiving rituximab maintenance therapy.
Summary
This study is the largest sample size (n=237) analysis of the efficacy of BR regimen in de novo EMZL. The main results include:
1 ) BR is a highly effective first-line treatment for EMZL, with an ORR of 93.2% and a CR rate of 81%, and the study included all extranodal sites; 2) Rituximab maintenance therapy resulted in longer PFS, But it did not affect OS;
3) Common infectious complications, including herpes zoster reactivation, pneumonia and influenza, were observed in BR treatment, which were not reported in previous MZL studies;
4) MALT-IPI lacks predictive value;
5) Few progression and death events occur within the first 24 months after BR treatment (POD24);
6) Symptom B is the only patient-related factor for poor prognosis in BR treatment
Literature
Juan Pablo Alderuccio, et al. An international analysis evaluating frontline bendamustine with rituximab in extranodal marginal zone lymphoma .Blood Adv. 2022 Feb 23;bloodadvances.2021006844