Shanghai, China—Recently, MicroPort Medical Technology Co., Ltd. (02160 4C Medical Technologies, Inc. (“4C Medical”), an associate company of .HK, hereinafter referred to as “Xintong Medical”), announced that its transcatheter mitral valve implantation device, the AltaValve™, has used its new generation transatrial The spaced, low outer diameter, fully retrievable delivery system has completed the first human clinical applications through humanitarian aid. The two cases of transseptal mitral valve replacement were performed by Dr. Vlasis Ninio, Director of Cardiology at Interbalken Medical Center in Thessaloniki, Greece.
Two patients were diagnosed with severe mitral regurgitation requiring treatment to relieve symptoms of heart failure. However, neither of these two patients is suitable for surgery due to physical reasons, and because of their special cardiac anatomy, they are not suitable for other transcatheter mitral valve treatment options such as the transapical approach. Therefore, the transseptal mitral valve is Replacement surgery became the only hope. Mitral regurgitation symptoms were significantly reduced in both patients postoperatively, and cardiac function improved. At present, the patient has been discharged from the hospital, and the quality of life after surgery has improved significantly.

▲AltaValve™ uses a unique supracircular valve device and atrial fixation design
< p>Vlasis Ninio commented after the surgery: “We are proud to have performed the world’s first AltaValve™ replacement using the next-generation transfemoral-transseptal access system, successfully and humanely for two patients who had no other treatment options. The most advanced rescue surgery. The AltaValve™ is easy to operate and can be applied to the wider mitral regurgitation patient population. The 4C Medical team has not only provided us with excellent clinical training and support throughout the process, but has also been dedicated to assisting patients. The success of the operation is inseparable from the efforts of the 4C Medical team.”
Transcatheter mitral valve replacement technology is very complex, and existing product solutions have many technical problems, including the possibility of causing left ventricular outflow tract obstruction , left ventricular dysfunction, equipment embolism and other complications, there is an urgent need for a product that can solve the above clinical pain points and provide more patients with safer and more effective medical solutions. Most of the existing transcatheter mitral valve replacement systems adopt the “transapical approach” design, but this technique requires a transapical approach with an incision of about 3-5 cm in the patient’s left anterior chest, which is more traumatic. Another “transfemoral-transseptal approach” requires only a tiny incision in the patient’s groin, which is less traumatic and the patient recovers faster, but it requires more complicated intervention routes and is more difficult to develop. big.
As a “transfemoral-transseptal” transcatheter mitral valve replacement product, AltaValve™ adopts a unique supracircular valve device and atrial fixation design, and is expected to become a global The first solution for mitral regurgitation that can be fixed only in the atrium, thus avoiding the anchoring and fixation difficulties in the currently known TMVR technology to the greatest extent, and can minimize the risk of left ventricular outflow tract obstruction, Applicable to more patients with mitral regurgitation. In addition, AltaValve™ is also the only fully recovered transatrial septal valve system known in the world that can achieve recovery function after complete release, enabling the operator to achieve more precise positioning and release during surgery.
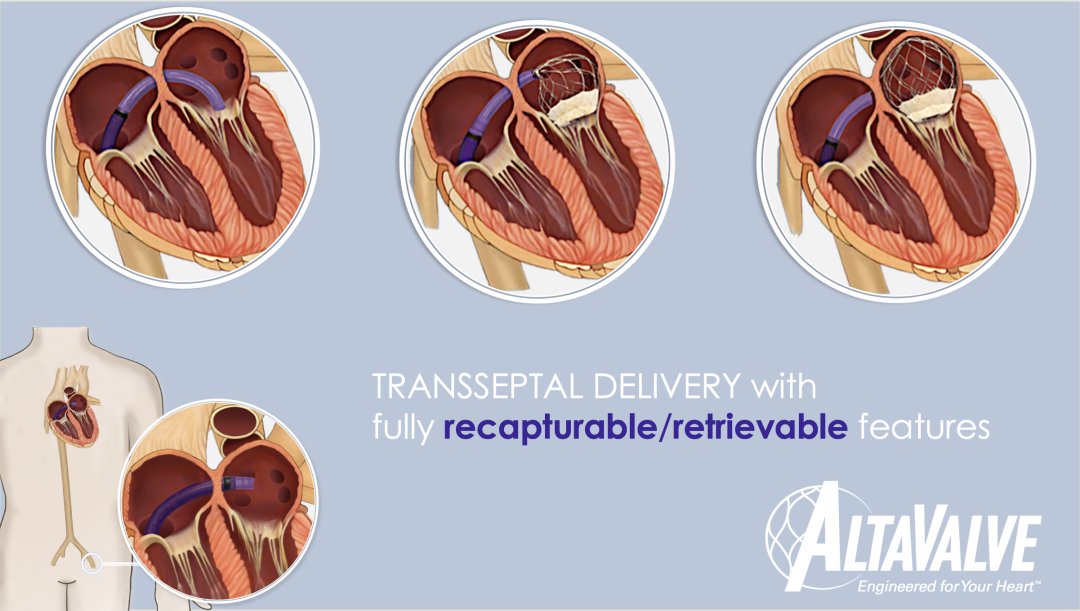
▲Schematic of AltaValve™ Implantation
Dr. Saravana Kumar, Chairman and CEO, 4C Medical “We are very excited about the clinical performance of the next-generation transseptal delivery system. 4C Medical has always been committed to optimizing treatment options, and we will continue to accumulate large-scale evidence-based data from AltaValve™ to help more patients with severe mitral regurgitation. At the same time, 4C Medical will continue to innovate in the field of structural heart disease and provide more solutions for the treatment of mitral and tricuspid regurgitation.”
Mitral regurgitation It is a common heart valve disease. The patient’s mitral valve cannot be fully closed, causing a part of blood to flow back into the left atrium from the left ventricle during ventricular systole. Severe mitral valve regurgitation can lead to heart enlargement and deformation or heart failure. In 2019, the number of patients with mitral regurgitation in the world was about 96.7 million, of which the number of patients in China accounted for nearly 1/9 of the total number of patients in the world, reaching 10.6 million. According to Frost & Sullivan, with the increasing global patient demand for transcatheter mitral valve repair/replacement products and the continuous emergence of more innovative technologies, it is estimated that by 2030, the global transcatheter mitral valve intervention therapy The market will be worth $17.4 billion, three to four times the size of the transcatheter aortic valve replacement market.
Mr. Chen Guoming, President of Xintong Medical, said: “As an innovative medical device company focusing on the research and development of transcatheter treatment technology for structural heart disease, 4C Medical’s research products cover the mitral valve and tricuspid valve. The field of valvular insufficiency. In September 2018, Xintong Medical participated in 4C Medical’s A round of financing and obtained the exclusive commercial rights of its mitral valve products in China. In March 2021, Xintong Medical made additional investment and became the Its largest shareholder has also obtained the exclusive commercial rights of its tricuspid valve products under development in China, thus further enriching the product line and forming a more complete and diversified product portfolio. In the future, Xintong Medical will continue to strengthen The cooperation between the two parties will provide safer and more effective medical solutions for structural heart disease patients worldwide.”