內容目錄
Precision therapy is a major trend in anti-cancer in the world. The development and release of special targeted drugs can fill the gap in the Chinese market.
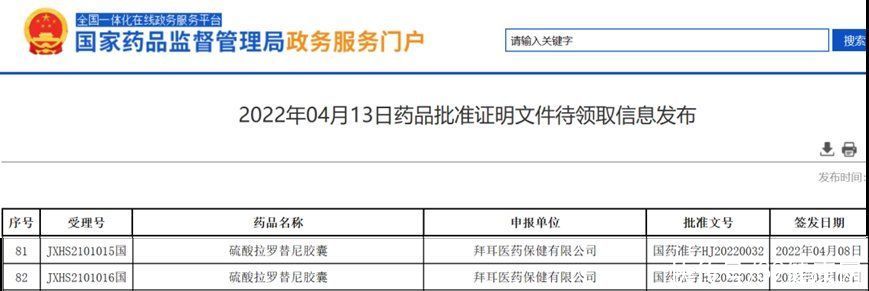
On April 13, 2022, the official website of the State Drug Administration showed that the “Larottinib Sulfate Capsules Marketing Application” submitted by Bayer has been approved. This means that China welcomesthe first pan-solid tumor targeted drug (TRK inhibitor) for NTRK gene fusion Larotrectinib is a boon for many Chinese cancer patients.
As early as 2018, larottinib was approved by the US FDA under accelerated approval. Becoming the first FDA-approved“broad-spectrum” targeted drug.
On May 26, 2021, larotrectinib was granted priority review by the State Drug Administration , I did not expect it to be approved in less than a year, which is of great significance to the Chinese pharmaceutical industry and cancer patients.
I. What is “magic” about larotrectinib
Larotrectinib is a specialized treatment A targeted drug with NTRK gene fusiontumor, and it is also the first “broad-spectrum” anti-cancer drug approved by the US FDA regardless of cancer type and only looking at NTRK fusion mutations targeted drugs.
NTRK gene can be fused with other genes, produce abnormal protein (TRK fusion protein), and then promote tumor growth and spread span>. Taking advantage of this feature, larotrectinib can strike precisely and produce anti-cancer effects.
NTRK gene fusion various solid tumors, including thyroid cancer, salivary gland cancer, breast cancer, colorectal cancer, lung cancer, pancreatic cancer, etc. Especially in rare cancers such as infantile fibrosarcoma, breast-like secretory carcinoma, and breast-secretory carcinoma, the incidence of NTRK gene fusion is as high as 90%.
Therefore, larotrectinib, as the first oralTRK inhibitor, has Broad-spectrum antitumor activity.
Second, filling the gap for domestic precision tumor therapy
Clinical trials are the only way for drugs to be approved, and larottinib’s 3 A large clinical trial has given gratifying results.
As of July 2020, 218 patients with NTRK gene fusion-positive cancers have been treated with larotrectinib, of which 206 are evaluable for efficacy,Includes 21 different tumor types.
The data showed that the overall response rate of larotrectinib was 75%75%, Among them, 45 patients had complete remission, 109 had partial remission, 33 had stable disease, and 13 had progressive diseaseBest Response. Among all evaluable patients, median time to optimal response was 1.8 months, median response< strong>Duration is 32.9 months.
Treatment-related adverse events during the trial Mainly grade 1-2, indicating that larotrectinib has excellent efficacy and safety.
Shanghai Pulmonary Hospital Affiliated to Tongji UniversityProfessor Zhou Caicun said that larotibide The official approval of NTRK in China is of great significance. It not only further improves the efficacy and prognosis of patients with NTRK gene fusion-positive tumors, but also fills the gap in the precision treatment of patients with NTRK gene fusion tumors.
3. What cancers can larottinib be used for?
Larotrectinib-suitable cancer types have increased from 17 to 21 according to latest trial data strong>, covering almost all solid tumor types.
Specifically including soft tissue sarcoma, infantile fibrosarcoma, primary central nervous system tumor, thyroid cancer, salivary gland cancer, lung cancer, colorectal cancer, melanoma, colon cancer, breast cancer, Gastrointestinal stromal tumor, osteosarcoma, cholangiocarcinoma, pancreatic cancer, congenital mesodermal renal cancer, primary unknown cancer, appendix cancer, liver cancer, prostate cancer and cervical cancer, etc.
Currently, the domestically approved indications are:
- carrying NTRK fusion gene and not Includes adult and pediatric solid tumors with known acquired resistance mutations;
- Locally advanced, metastatic disease, or surgical resection of adult and pediatric solid tumors that may lead to serious complications;
- No satisfaction Replacement therapy or failure of prior therapy in adults and children with solid tumors.
Fourth, you may want to know these questions
Q1: Is there any drug resistance?
As a targeted drug, larotrectinib also has drug resistance, which is mainly related to the patient’s NTRK gene New mutationsrelated. For the resistance of larotrectinib, the second-generation TRK-targeted drugs have come out. If new resistance sites are discovered in the future, scientists still need to solve them.
Q2: Do I need genetic testing?
Patients must undergo genetic testing before using targeted drugs. At present, there are four main detection methods for NTRK gene fusion:
- Immunohistochemistry (IHC): Widespread, low cost, reimbursement, but limited specificity, can only be used as primary screening.
- Fluorescence in situ hybridization (FISH): It has high sensitivity and specificity, uses few samples, and is highly sensitive to tumor purity. Low requirements, but requires fluorescence microscopy and cannot identify fusion partners.
- Reverse Transcription-Polymerase Chain Reaction (RT-PCR): High sensitivity and specificity, can detect Gene fusions expressed at the RNA level are less expensive. The disadvantage is that the target sequence must be known, and new fusion partners cannot be detected.
- DNA and RNA-based Next Generation Sequencing (NGS): High sensitivity and specificity, can detect To a novel fusion partner, multiple targetable therapeutic targets can be detected simultaneously. The disadvantage is that it takes a long time (1-3 weeks) and the cost is higher.

Q3: When will it be available? How much does that cost?
Generally speaking, after a new drug is approved for marketingnot yet available for direct sale< span>, need to conduct trial observation to record the trial effect of the drug. Only through the trial and observation stage can merchants conduct mass production and put them on the shelves. From approval to purchase, takes approximately 3-5 months.
As for the price, the price of larotrectinib in the US is $32,800 per month, about RMB 2.5 million per year. At present, the domestic price has not been determined, and I hope it will not be too expensive, so that more patients can afford it.
The launch of new anti-cancer drugs is great news for cancer patients and their families. I hope more anti-cancer methods will appear in the future to benefit the common people.
# Yaozero Zero Plan# #Wonderful Knowledge Season#
References:
[1]“Unlimited The targeted drug larottinib has been approved in China, and the precision treatment of domestic tumors will be a sharper edge! .Medical Tumor Channel, 2022-04-17
[2] The clinical data of larotrectinib in the treatment of lung cancer is eye-catching, filling the gap for precision treatment of lung cancer. China Medical Tribune, 2022-04-13< /p>
[3]Hong DS, Shen L, Van Tilburg CM, et al. Long-term efficacy and safety of larotrectinib in an integrated dataset of patients with TRK fusion cancer. J Clin Oncol. 2021;39(suppl 15): abstr 3108. doi: 10.1200/ JCO.2021.39.15_suppl.3108
Reprinting is prohibited without the author’s permission