On March 18, Beijing time, the official website of the Geneva Medicines Patent Pool (MPP) announced that it has signed agreements with 35 companies that are allowed to imitate the production of Naima, one of the ingredients of Pfizer’s new crown oral drug Paxlovid. Tevir (nirmatrelvir) API or preparation. On the 17th, the market rumors that Pfizer’s new crown oral drug generic license was reported by many media was officially confirmed.
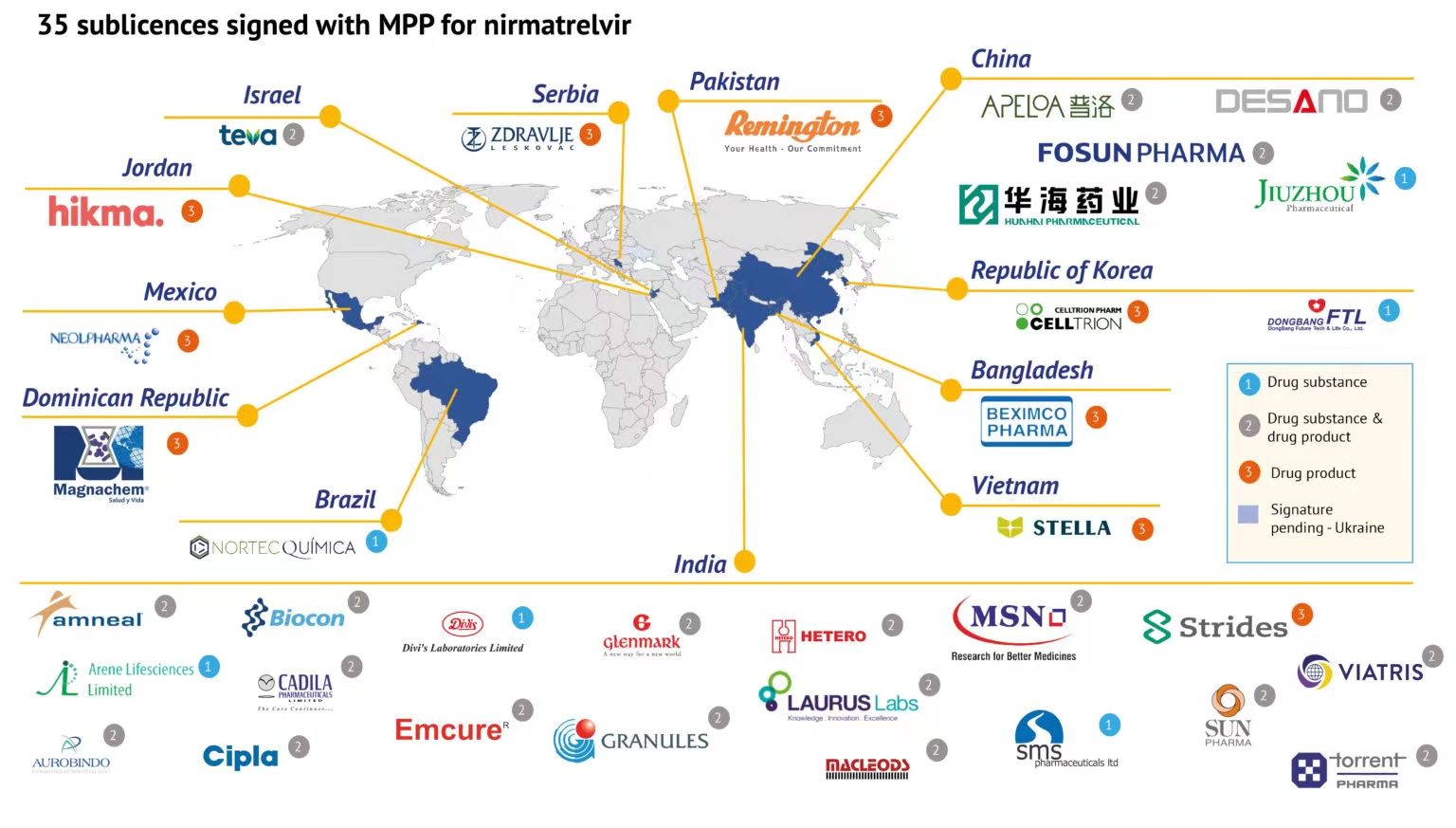 Photo source: MPP
Photo source: MPP
The reporter noticed that the list includes 5 Chinese pharmaceutical companies , respectively, Fosun Pharma, Huahai Pharma, Pro Pharma, Jiuzhou Pharma, Shanghai Desano, among which Jiuzhou Pharma only produces APIs, and the rest can produce both APIs and preparations.
According to the official website, the Medicines Patent Pool is a public health organization supported by the United Nations, dedicated to increasing access to life-saving medicines for low- and middle-income countries and promoting drug development.
There are early signs of this authorization. On January 18, Pfizer announced that it had signed a voluntary license agreement with the Medicines Patent Pool (MPP) to help expand its accessibility in 95 low- and middle-income countries, which accounted for about 53% of the total. World Population. But the news did not reveal which companies were involved.
Paxlovid is an oral small molecule new crown treatment drug, specifically, the drug is a combination of PF-07321332 and ritonavir. On February 11, the State Food and Drug Administration announced that according to the relevant provisions of the “Drug Administration Law”, in accordance with the special drug approval procedures, emergency review and approval, and conditional approval of Pfizer’s new coronavirus treatment drug naimatevir tablets / Tonavir tablet combination package (ie Paxlovid) import registration.
This is not the first time that a Chinese pharmaceutical company has been authorized to imitate a new crown oral drug. On January 20, the Medicines Patent Pool (MPP) announced through its official website that it has signed agreements with 27 pharmaceutical companies to allow them to produce and supply Merck’s oral anti-new coronavirus drug Molnupiravir to 105 low- and middle-income countries or regions around the world to promote Affordability and accessibility of the drug globally. Five Chinese pharmaceutical companies, including Fosun Pharma, Borui Pharmaceutical, Shijiazhuang Longze Pharmaceutical, Shanghai Desano, and Langhua Pharmaceutical, are among them. The first four are licensed to produce both APIs and finished drugs. Production of APIs.