(People’s Daily Health Client Reporter Wang Zhenya) “In 2021, the State Food and Drug Administration approved a total of 11,314 medical device registrations, and 35 innovative medical devices were listed.” The State Food and Drug Administration released the 2021 medical device registration big data and Policy interpretation of the 2021 list of innovative medical devices.
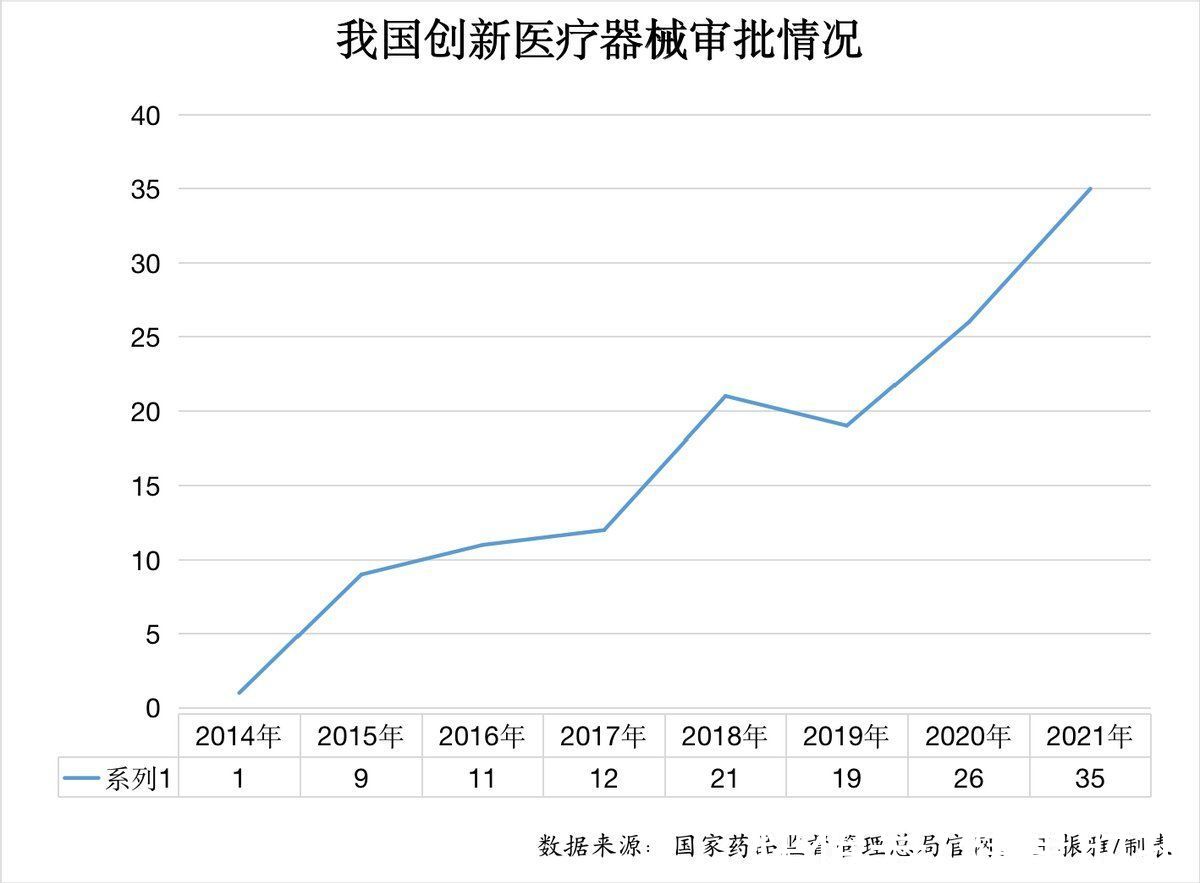
People’s Daily health client reporters found that my country’s innovative medical devices have increased from 1 in 2014, to 26 in 2020, and then to 35 in 2021, showing a rapid increase every year, industry insider Xie Yijiong told A reporter from the People’s Daily Health Client, 35 innovative medical devices were launched in one year, which has hit a record high.
The “green channel” of medical devices
Innovative medical devices are patented, technical It belongs to the domestic first, international leading medical device with significant clinical application value. The State Food and Drug Administration will give priority to review and approval under the premise that the standards and procedures are not reduced. The green channel for innovative medical devices started in 2014.
In February 2014, the State Food and Drug Administration issued the “Special Approval Procedures for Innovative Medical Devices (Trial)” (revised to “Special Examination Procedures for Innovative Medical Devices” in 2018), setting a fast track for innovative medical devices. Approval channel to encourage the research and development of innovative medical devices. From the data, 1 innovative device was approved in 2014, 9 in 2015, 11 in 2016, 12 in 2017, 21 in 2018, 19 in 2019, 26 in 2020, and 35 in 2021.
The number of innovative medical devices approved for listing is increasing year by year, but compared with the overall medical device approval data, the number of innovative medical devices listed each year seems to be pitiful. Xie Yijiong introduced that this is due to its high “threshold”. As of the end of 2021, there have been more than 2,200 applications for special approval for innovative medical devices, and 134 products have been approved for marketing, with an approval rate of only 5.2%.
Expediting the approval process and promoting the early listing of medical devices
According to the statistics of the State Drug Administration, the time for obtaining the registration certificate of the State Drug Administration for products that have entered the special approval process for innovative medical devices is longer than that of similar products. The average reduction for other products was 83 days. Xie Yijiong told reporters that the period for obtaining the medical device certification has been greatly shortened, and the product can enter the market as soon as possible, which undoubtedly increases the competitiveness of the product and seizes the market opportunity.
For example, for Mingfeng ScintCare PET/CT, it takes 9 months from application to get the certificate, and the speed of getting the certificate is very fast; Yongxin Medical SPECT takes 13 months from application to get the certificate, which is also much lower than The usual 24 months.
On January 13, 2021, Lifeflow™ iliac artery bifurcation stent system independently developed by Lifetech, as the first medical device for intracavitary reconstruction of the internal iliac artery independently developed in my country, has It took 41 months for the green channel to be approved.