According to the website of the State Food and Drug Administration, on the 13th, the State Food and Drug Administration approved the registration application of 5 new crown antigen detection kit products.
The 5 companies include Beijing Wantai Bio-Pharmaceutical Co., Ltd., Beijing Rejing Biotechnology Co., Ltd., Tianjin Boaosaisi Biotechnology Co., Ltd., Chongqing Mingdao Jiexi Biotechnology Co., Ltd., and Beijing Lepu Diagnostics Technology Co., Ltd.
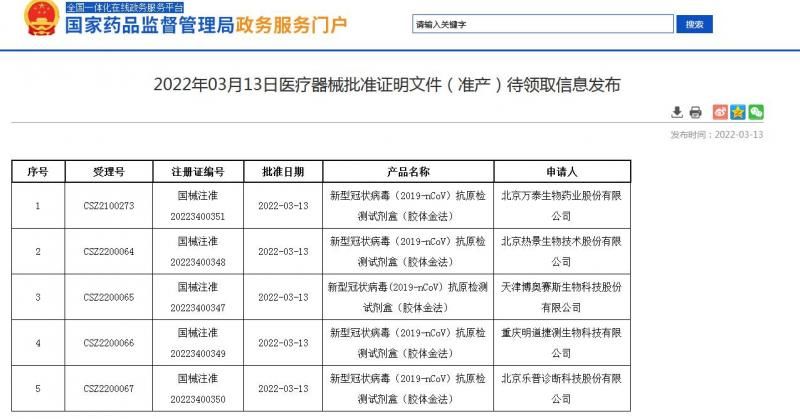
Source: State Food and Drug Administration website
Since then, 10 new crown antigen detection kit products have been launched.
On the 11th, the Comprehensive Team of the Joint Prevention and Control Mechanism of the New Coronavirus Pneumonia of the State Council issued the “Notice on Printing and Distributing the Application Plan for the New Coronavirus Antigen Detection (Trial)”, showing that after research, it was decided to increase the antigen detection as the basis for nucleic acid detection. Replenish.
Subsequently, the medical device approval documents (changes) pending receipt published on the website of the State Food and Drug Administration showed that Beijing Huaketai Biotechnology Co., Ltd., Nanjing Novizan Medical Technology Co., Ltd., and Beijing Jinwofu Bioengineering Technology Co., Ltd. Five new coronavirus antigen detection kits from the company, Shenzhen Huada Yinyuan Pharmaceutical Technology Co., Ltd., and Guangzhou Wanfu Biotechnology Co., Ltd. have passed the registration information change of the Food and Drug Administration.
(Sino-Singapore Jingwei APP)