On March 12, the Red Star Capital Bureau noticed that the information on the medical device approval certificate (change) published on the website of the State Food and Drug Administration showed that Wanfu Bio (300482.SZ), Huada Gene (300676. SZ) subsidiary Shenzhen Huada Yinyuan Pharmaceutical Technology Co., Ltd., Beijing Jinwofu Bioengineering Technology Co., Ltd., and Nanjing Novizan (688105.SH) Medical Technology Co., Ltd. The 4 new crown antigen detection kits developed by 4 companies have passed The registration information of the Food and Drug Administration has been changed. In addition, on March 11, the State Food and Drug Administration announced that Beijing Huaketai Bio’s new crown antigen product self-test application application was changed.
Since then, five COVID-19 antigen self-test products have been officially launched.
Antigen rapid detection kit according to Vision China
Yes According to professional analysis, the core of this “registration change” is that the detection reagent is no longer limited to “only used by professional and technical personnel”. This may also mean that relevant testing reagents will be available for other scenarios such as home self-testing.
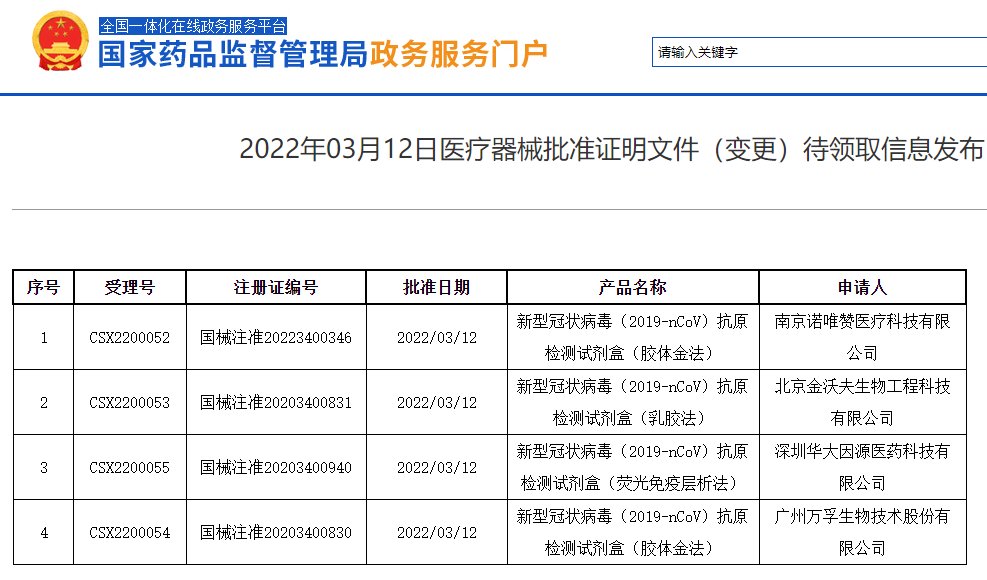
According to the official website of the State Drug Administration
< p> Just the day before, the National Health and Medical Commission announced that the comprehensive team of the Joint Prevention and Control Mechanism of the State Council decided to add antigen detection to the nucleic acid detection as a supplement, and organized the formulation of the “New Coronavirus Antigen Detection Application Plan (Trial)” ” (hereinafter referred to as the “Plan”).
The “Plan” stipulates the applicable population of antigen testing: first, those who go to primary medical and health institutions for treatment, with symptoms such as respiratory tract and fever, and have symptoms within 5 days; second, those who are quarantined and observed, including Home isolation observation, close contact and sub-close contact, entry isolation observation, people in closed control areas and control areas; the third is community residents who need antigen self-testing. Among them, the plan proposes that community residents who have self-test needs can purchase antigen detection reagents for self-test through retail pharmacies, online sales platforms and other channels.
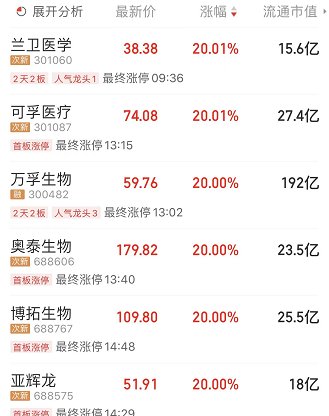
As soon as the “Plan” came out, the capital market responded on Friday, and the market closed on Friday, March 11, and the COVID-19 testing sector rose in a straight line. Lanwei Medical (301060.SZ), Kefu Medical ( 301087.SZ) and other 6 stocks “20CM” daily limit, Mingde Bio (002932.SZ), Jiu’an Medical (002432.SZ) and many other stocks daily limit, Oriental Bio (688298.SH), Novozan rose more than 17 %.
For the market potential of new coronavirus antigen detection reagents, Southwest Securities Research Report shows that the main new coronavirus antigen home self-test kits The main markets in the future are expected to come from the United States, the European Union, Japan and China. According to the total population and the average number of tests, the global market potential is expected to exceed 50 billion US dollars, of which the US market occupies the majority and the domestic market is expected to reach 8.4 billion US dollars.
Zhongtai Securities believes that the predicted domestic monthly market size may be 17.7 billion-26.6 billion yuan. Taking into account the purchases of residents and enterprises at their own expense, it is expected that the purchase demand for new crown antigen detection products is expected to further increase.
Red Star News Comprehensive Report Editor Yang Cheng
(Download Red Star News, there will be prizes for reporting materials!)
