內容目錄
Farewell to 1.2 million? The country’s first universal anti-cancer magic drug was approved for clinical trials!
In June 2021, a 1.2 million anti-cancer drug-Aquilence injection was approved for marketing in China, which attracted widespread attention, even though many people Shouting is not enough, butin less than a year, more than 100 patients have been treated, and the revenue has reached 120 million yuan.
In the face of cancer, some people are still willing to give everything in exchange for life. Can’t the price of the sky-high anti-cancer drug be reduced? Perhaps a new drug recently approved can change this situation.

First, the first in China! The universal anti-cancer magic drug was approved for clinical trials
In the past, the anti-cancer magic drug was expensive in “private customization”. On March 17, it was independently developed by a Chinese company< strong>The CTA101 UCAR-T cell injection of CTA101 has obtained the implied license for clinical trials of the State Food and Drug Administration, which is the first UCAR-T cell therapy product approved in my country. It is a general-purpose CAR-T therapy product.
CTA101 UCAR-T cell injection is mainly used for the treatment of adult relapsed and refractory B-cell acute lymphoblastic leukemia. This is the first UCAR-T cell therapy innovative drug approved in my countrywith Chinese independent intellectual property rights An important step has been taken in the innovative drug, which has a milestone significance!
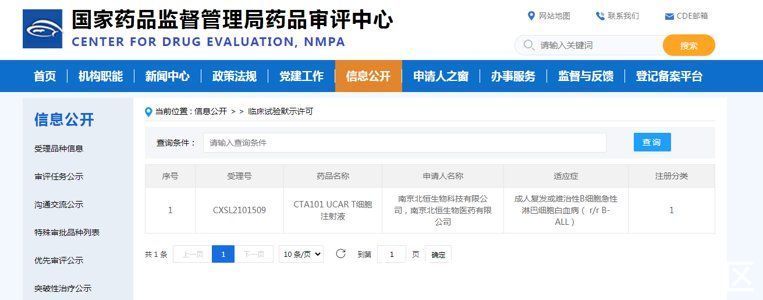
Before this, there are many anti-cancer drugs that have been marketed internationally. What are the specific ones? Look down together.
Second, one shot “kills” cancer cells and takes stock of the anti-cancer magic drugs that have been marketed before
CAR-T therapy is an emerging immune The full name is chimeric antigen receptor T cells. It refers to the isolation of T cells in patients from blood, and the in vitro genetic manipulation of them by viral and non-viral transfection.
After the CAR-T cells are generated, they are propagated in the laboratory to produce a large number of CAR-T cells that can be used for treatment, and then the cells are returned to the patient’s circulatory system , to help identify specific antigens on tumor cells, so as to play an anti-tumor effect.
There are currently 7 autologous CAR-T therapies on the global market, which have been criticized for a long time The 1.2 million yuan anti-cancer drug, even the cheapest one…
1, Kymriah ($475,000/needle)
span>
Approved for marketing on August 30, 2017, it is mainly used for the treatment of refractory B-cell acute lymphoblastic leukemia that has relapsed after at least second-line therapy.
2, Abecma ($437,900/pin)
March 26, 2021 CAR-T therapy for the treatment of multiple myeloma was approved for marketing.

3, Breyanzi ($410,300/pin)
Approved for marketing on February 5, 2021, for B lymphoma, follicular center lymphoma, diffuse large B cell lymphoma, primary mediastinal enlargement CAR-T therapy for the treatment of B-cell lymphoma and primary mediastinal large B-cell lymphoma.
4. Tecartus ($373,000/pin)
Oct 28, 2020 The CAR-T therapy drug for the treatment of mantle cell lymphoma was approved to be marketed in Japan.
5. Yescarta ($373,000/pin)
Oct 18, 2017 Approved for the treatment of adult large B-cell lymphoma after at least two other treatment modalities have failed. In March 2021, the drug was also approved for the treatment of adults with relapsed/refractory follicular lymphoma after two or multiple lines of systemic therapy.
6. Requilense Injection (1.29 million RMB/needle)
It was approved for marketing on September 3, 2021, for the treatment of diffuse large B-cell lymphoma CAR-T therapy.

7. Achilles injection (1.2 million RMB/needle) )
Approved for marketing on June 22, 2021 as a CAR-T therapy for the treatment of adult relapsed and refractory large B-cell lymphoma.
Compared with these sky-high-priced drugs, the new UCAR-T drugs developed in my country have great potential.The big advantage has brought new hope to countless patients.
3. The new drug is approved, one collection saves 100 people, and the price is cheaper
1. Say goodbye to personal customization, one collection saves 100 people
Autologous CAR-T therapy requires the extraction of T cells from the patient first, which requires 1:1 customization. This method is not only expensive, but also requires a certain amount of time.
Universal CAR-T therapy is a good solution to these dilemmas, it no need to extract T cells from the patient’s own , the T cells of healthy people can be directly used, and after being transformed by gene editing technology, they can be cultured on a large scale, and a healthy donor can prepare hundreds of “spots”.
2, the price is cheaper, or reduced to 1/10 of the original price
< p>
Compared with autologous CAR-T therapy, the price of universal CAR-T therapy is expected to be 1/10 of the original price, Allow patients to obtain greater benefits. “Times Finance” has also interviewed professionals, predicting that the general-purpose CAR-T therapy is even expected to be reduced to 5,000 to 30,000 yuanNo Wait.
3. Immediate use, stable curative effect, benefit more Human
Universal CAR-T therapy has the characteristics of immediate availability and can be administered to patients at the first time /span>, to avoid delaying the course of the disease. And the quality of T cells provided by healthy people is relatively stable, which is undoubtedly a huge boon for some patients who cannot use autologous CAR-T cells.
With such a good anti-cancer drug, can it be treated for all cancers? Can cancer be completely eliminated?
4. Can the anti-cancer drug cure all cancers?
Professor Ying Zhitao, Deputy Chief Physician of Peking University Cancer Hospital said that CAT-T therapy is effective for gastrointestinal tumors, gynecological tumors and other entities. The effect of tumor treatment is far less than that of blood tumors. Even patients with diffuse large B-cell lymphoma who have been treated have a chance of recurrence. According to clinical statistics, about 30% to 40% of patients can survive long-term disease-free, and about 60% of patients will still relapse.
For CAR-T therapy, we must have a correct attitude. Although it is effective for some special cancers, it is not without side effects. The most common side effect of CAR-T therapy is cytokine release syndrome, which can cause high fever, fatigue, nausea, hypotension, and cardiac insufficiency and other symptoms.
On the way to treat cancer, many researchers are working hard to study it. I believe that more and more cancer “nemesis” will be developed in the future! Let people stop talking about discoloration about cancer.
References:
[1] “The first in China! This company’s “spot” can beat cancer”. Nanjing Jiangbei New District. 2022.3.26
[2] “What is the expensive CAR-T therapy? “. Medical Mai Tong Hematology. 2022.3.28
[3] “One shot to clear cancer cells! The 1.2 million yuan anti-cancer therapy may be reduced to 5,000 yuan in the future, and the patent for survival is not for the rich”. Time Times.2021.09.18
Reprinting is prohibited without the author’s permission
span>